Introduction
The 4th commonest malignancy in females is cervix cancer [1], with preponderance between those of socioeconomic low level. Magnetic resonance imaging (MRI) is the most prevalent tool in cervical cancer work-up. The International Federation of Gynaecology and Obstetrics (FIGO) classification [2] depends on clinical examination for tumour staging. Currently, it implies the use of imaging tools as an adjunct for staging [3]. Sampling error, difficulty in determining invasion of the parametrium, and accurate tumour size calculation are sufficient causes, making histopathology alone inefficient. Those items help in choosing the best treatment option between surgery and chemotherapy [4]. Combined high-resolution MRI and magnetic resonance spectroscopy (MRS) could differentiate preinvasion from early invasive cervical tumours. Multiple studies showed resonances from mobile triglycerides, confirmed by biopsy samples in cases of invasive cervical carcinoma [5]. Limitations of MRS include field strengths and its spectral resolution, and inhomogeneity of the magnetic field resulting in line-broadening issues [6]; these effects can be abandoned by applying a 54.7° spinning angle at the main field [7], an effect named magic angle spinning (MAS) MRS producing excellent spectrum resolution, yet at high spin, some cellular structural degradation could ensue. This pilot study aimed to assess the diagnostic accuracy of lipid (1.3 ppm) peak in predicting the high-grade cervical cancer to highlight the need for more advanced non-invasive imaging techniques that can accurately differentiate the low- and high-grade cervical cancer for better patient management. As a secondary aim, we investigated the difference between low- and high-grade cervical cancer groups regarding lipid and choline peaks.
Material and methods
Before the beginning of the study, all patients had signed a local approval and informed consent form regarding the institutional review board (IRB) rules with a reference number ZU-11060. We followed the Helsinki concepts and STARD guidelines in conducting the research.
Study design and population
This prospective pilot study included 20 patients, with an age range from 46 to 70 years and a mean age of 57.5 years. They were referred from the Department of Obstetrics and Gynaecology to the Department of Diagnostic Radiology during the period from March 2022 to March 2023. All the images used in this study were collected from our existing PACS database.
Patients who underwent sonography that detected a cervical mass, or presented with a cervical mass at local examination with pathologically proven cervical carcinoma were included. Patients incompatible for MRI examination, e.g. pacemaker, metallic prosthesis (n = 1), or patients unwilling to complete the study, patients with claustrophobia for MRI (n = 3), patients with indeterminate pathological results, and cases with suboptimal MRI and MRS images (n = 2) were also excluded. All patients were subjected to the following: i) personal history (name, age, occupation…), ii) local gynaecological examination, iii) sonographic (transvaginal) examination, if they were not done before MRI exam, and iv) MRI and MRS of the cervix. The flow chart of the research process is illustrated in Figure 1.
Technique of MRI and MRS
Scan protocol and parameters
The MRI scanner (superconducting magnet) used in the current study was a 1.5 T Achieva (Philips Medical Systems). Patients were dressed in light cotton gowns. The exam was performed while patients presented supine with their feet first. Imaging acquisition was done using a dedicated 16-channel pelvic phased array coil, positioned on the pelvis. One hour prior to the exam patients had been asked to void. To reduce bowel motion artifacts, 10 mg of hyoscine N-butyl bromide (Buscopan) was injected intravenously. First, the scout was done, and it extended from the level of L4-5 (level of renal hilum) to the level of ischial tuberosities to ensure the covering of the cervix and its surrounding structures. Then, the chosen MRI protocol was performed. The scanning protocol and parameters are shown in Table 1.
Table 1
Parameters of cervical MRI scan sequences
1H-MRS was acquired using a single-voxel point resolved spectroscopy (PRESS) sequence, with a repetition time (TR) of 1500 ms and echo times (TE) of 28 and 144 ms. A voxel (average volume 18 × 18 × 18 mm³) was positioned in the centre of the lesion, strictly avoiding contamination from nearby tissues and necrotic areas. Short TE (28 ms) was used for metabolite quantification, while long TE (144 ms) was used for the detection of lactate (Lac) (its signal can be hidden when a large lipid signal resonating at 1.28 ppm is also present). Automated shimming to acquire superb spectral quality and reduction of total acquisition time was applied.
The water signal was suppressed by using chemical selective saturation (CHESS) pulses. The total scan time (MRI + 1H-MRS) was about 20 min.
Image analysis
Two experienced radiologists with 10 years of experience in gynaecological imaging blinded to the results of the histopathological examinations independently analysed all the conventional MRI images and MRS (lipid and choline) metabolites spectra and assigned each lesion a FIGO stage according to 2018 classification [8], and any disagreement was resolved by a third radiologist with 15 years of experience in gynaecological imaging. Spectra were analysed for 17 metabolites, as shown in Table 2. Metabolites with overlapping or very close resonance are given their sum (for example tCho = GPC + PCho and Glx = Glu + Gln). We used the receiver operating characteristics curve (ROC) to calculate the best cutoff of lipid peak (1.3 ppm) for predicting high-grade cervical cancer and the Mann-Whitney U test to compare both groups regarding MRS metabolites.
Table 2
The 17 metabolites included at the MRS spectra analysis
Histopathology (Reference test)
All patients underwent a diagnostic biopsy from the cervix with determination of pathologic subtypes and lesion grading. Results were reviewed by two experienced pathologists. The lesions were divided into low- and high-grade cervical lesions according to the shape and the degree of differentiation of the malignant cells in the specimen. The low-grade lesions involved cervical intra-epithelial neoplasia grade 1 (CIN1), while high-grade lesions included CIN2, CIN3, and invasive carcinoma – either squamous cell carcinoma or adenocarcinoma [9].
Statistical analysis
Data analysis was conducted using the Jamovi software version 2.3.26. Categorical data were described using numbers and frequencies. We assessed the normality of the continuous data by Shapiro-Wilk test. For normally distributed data, means and standard deviations, and for non-normally distributed data, median with interquartile range were used to describe the continuous data. We utilised the Mann-Whitney test to compare the low- and high-grade cervical cancer groups regarding MRS metabolites. For calculating the cutoff value of lipid peak (1.3 ppm) to predict high-grade cervical cancer, the ROC curve was utilised. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy with a confidence interval of 95% were calculated. The p-value of less than 0.05 was considered statistically significant.
Results
The current study included 20 female patients with pathologically proven cervical cancer. The mean age of the studied patients was 59.25 ± 5.87 years. Nine cases were low-grade, and 11 were high-grade cervical carcinoma. The rate of high-grade cervical carcinoma was 55%. The most prevalent histological type was squamous cell carcinoma, found in 14 cases (Table 3).
Table 3
Patients’ basic characteristics of the studied groups
Comparison between the low- and high-grade groups regarding the MRS metabolites
As shown in Table 4, there was a statistically significant difference between the 2 groups regarding lipid peak (0.9 ppm), lipid peak (1.3 ppm), and choline peak (p-value of 0.025, 0.001, and 0.023, respectively). The lipid/choline ratio did not differ between both groups (p-value of 0.271).
Table 4
Comparison between low- and high-grade cervical cancer groups regarding MRS metabolites
| Low-grade | High-grade | p-value | |
|---|---|---|---|
| Lipid peak (1.3 ppm), median (IQR) | 20.6 (9.45) | 52.9 (21.5) | 0.001* |
| Lipid peak (0.9 ppm) mean ± SD/ Median (IQR) | 14.88±2.33 | 33.9 (18.9) | 0.025* |
| Choline peak (ppm) median (IQR) | 7.58±1.45 | 17.9 (10.9) | 0.023* |
| Lipid/choline ratio Median (IQR) | 1.79 (0.75) | 1.8 (0.87) | 0.271* |
Diagnostic performance of lipid peak metabolite (1.3 ppm) to predict high-grade cervical carcinoma
MRS lipid (1.3 ppm) showed high diagnostic performance in predicting high-grade cervical carcinoma. Lipid peak (1.3 ppm) showed a sensitivity, specificity, and accuracy of 100%, 77.8%, and 90%, respectively. The diagnostic accuracy of the lipid peak (1.3 ppm) is described in Table 5.
Analysis of the ROC curve
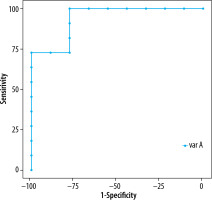
Using the ROC curve, the optimal cutoff value of lipid peak (1.3 ppm) to predict high-grade cervical carcinoma was 29.9 (Figure 2) with an AUC of 0.939 (Table 5).
Figure 2
ROC curve of the diagnostic accuracy of lipid (1.3 ppm) in predicting the high-grade cervical cancer
Some of our cases are illustrated in (Figures 3-5).
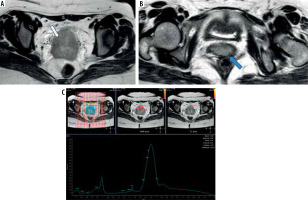
Figure 3
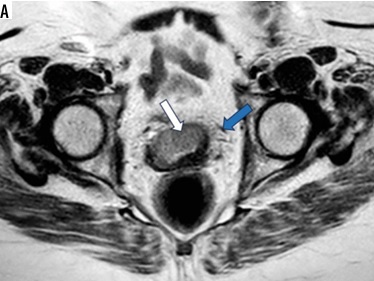
A 60-year-old female patient presented with dyspareunia and US cervical mass. A) Axial T2WI shows a moderatley well-defined cervical soft tissue mass displaying intermediate signal intensity (SI) with loss of stromal low SI from 9:00 to 1:00 o’clock (white arrow) associated with parametrial extension on both sides (blue arrow). It measures about 40 × 20 mm (FIGO IIB). B) Magnetic resonance spectroscopy curve shows high lipid and choline peaks. Histopathology reveals high-grade cervical squamous-cell carcinoma
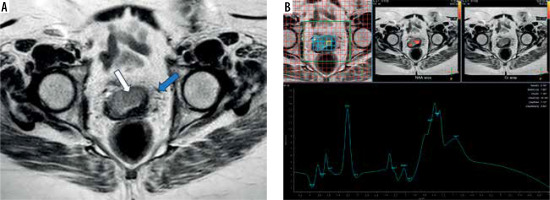
Figure 4
A 45-year-old female patient presented with vaginal bleeding on sexual intercourse and US cervical mass. A) Sagittal T2WI shows a poorly defined cervical soft tissue mass (white arrow) displaying intermediate signal intensity (SI) associated with loss of cervical stromal low SI and extending to the posterior upper vaginal wall (blue arrow). It measures about 65 × 60 mm (FIGO IIB). B) Magnetic resonance spectroscopy curve shows high choline and moderate elevation of lipid peaks. Histopathology reveals low-grade cervical adenocarcinoma on multiple specimens
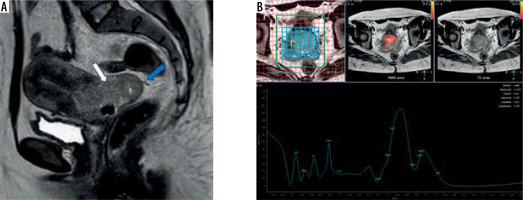
Figure 5
A 34-year-old female patient presented with abnormal intermenstrual bloody discharge and US cervical ill-defined mass. A-B) Axial T2WIs show a moderately well-defined cervical soft tissue mass displaying intermediate signal intensity (SI) with circumferential loss of stromal low SI (white arrow) and extending to the lower vaginal third (blue arrow). It measures 60 × 60 mm. C) Magnetic resonance spectroscopy curve shows high lipid and moderate elevation of choline peaks. Histopathology reveals high-grade squamous-cell carcinoma
Discussion
The current pilot study is an attempt to provide literature on the clinical impact of in vivo MRS in predicting the grade of cervical carcinoma. This functional and advanced MRI technique can decrease biopsy rates and has the potential to assess the cancer grade in a non-invasive way. Accurate discrimination between low- and high-grade cervical carcinoma is crucial for better decision-making and management. Furthermore, high-grade cervical carcinoma needs surgery in addition to chemo- or radiotherapy with a close follow-up, while low-grade cervical carcinoma may require minimally invasive intervention. Moreover, MRS metabolites can be used in the assessment of post-treatment response for better patient outcomes [10].
The current pilot study is in agreement with what is already known in the previous literature about the diagnostic performance of MRS lipid metabolites in discrimination between low- and high-grade cervical carcinoma. Nine low-grade and 11 high-grade cases were recruited. Squamous cell carcinoma was the most prevalent detected histological type. A lipid peak (1.3 ppm) of 29.9 was the optimal cutoff value for predicting high-grade cervical carcinoma with sensitivity, specificity, and accuracy of 100%, 77.8%, and 90%, respectively. Moreover, there was a statistically significant difference between low- and high-grade cervical cancer groups concerning the MRS different metabolites (lipid 0.9 ppm, lipid 1.3 ppm, and choline). These findings were not surprising and could be explained by the fact that there was an increase in the amount of unsaturated fatty acids in poorly differentiated tumours. However, the presence of more than 50% of high-grade cases in the current study might be a potential bias in calculating the diagnostic accuracy of MRS metabolites in predicting high-grade cervical carcinoma because the patients were recruited from a single gynaecological-oncological institution with many advanced cases.
To the best of our knowledge, there is a paucity of previously published studies to assess the diagnostic performance of the MRS metabolites in differentiating between low- and high-grade cervical cancer. This pilot study is an attempt to validate the MRS lipid metabolites in diagnosing high-grade cervical cancer cases and to calculate an optimal cutoff of lipid peak (1.3 ppm). However, many studies evaluated the clinical utility of MRS in cervical cancer and reported elevated levels of fatty acids in the cervical cancer, especially adenocarcinoma histological type [11-16]. A previous study [13] validated this technique using in vivo and ex vivo profiles and reported a sensitivity and specificity of 77.4% and 93.8%, respectively, for CH2 in vivo, the sensitivity was 100% and specificity was 69% for CH2 ex vivo, supporting the current study findings. Our pilot study reported a 29.9 cutoff for lipid peak (1.3 ppm) for differentiating between low- and high-grade cervical carcinoma. However, there was no recorded cutoff in the previously published reports to support or contradict our results.
Regarding the difference in MRS lipid metabolites between both groups, Mahon et al. [13] reported a significant difference between normal, CIN, and cervical cancer groups regarding lipid (0.9 ppm) and lipid (1.3 ppm) (p < 0.0001), which is in line with our study. Conversely, a team in Taiwan [17] assessed the clinical value of proton MRS in cervical carcinomas and prediction of poor prognostic human papillomavirus (HPV) genotypes, and recorded no significant difference between the well- and moderate/poorly differentiated cervical carcinoma regarding lipid (0.9 and 1.3 ppm) with p-values of 0.245 and 0.361, respectively. This discrepancy might be attributed to the fact that their study included 52 cases and using a 3 Tesla machine.
Regarding choline peak, Mahon et al. [13] and Lin et al. [17] reported that both groups did not differ in total choline peak, with p-values of 0.68 and 0.918, which is not in line with our study. This can be explained by the fact that the choline peak can be variable in different degrees of tumour differentiation and our study enrolled a small group. Previously conducted studies recorded an association of Cho level with high-grade and large tumours. Moreover, there is a significant association between the presence of choline and the absence of necrosis [18].
Increases in lipid peaks (0.9 and 1.3 ppm) resonances are demonstrated in the growth and invasiveness of the tumour cells [19]. The actual proof for their origin in cytoplasmic or membranous compartments is still a matter of debate. Without direct electron microscope evidence, the MR evidence supporting the membrane microdomain hypothesis [20] was based on spectrum similarities and relaxation characteristics between lipoproteins, membrane preparations, and cell suspensions. Furthermore, cytoplasmic lipids have been demonstrated as a source of lipid peaks [21,22]. Limited studies were conducted to investigate the association of lipid peak resonances and cell proliferation and apoptosis.
Clinical implications
MRS lipid peaks can be used in clinical practice as a valid imaging tool for assessing the grade of cervical cancer. Furthermore, it might be applicable for pretreatment staging, planning the optimal therapy, and follow-up. Recent studies investigated its clinical use in the evaluation of the treatment response for better patient outcome. DWI provides functional information on the tumour nature, tumour extension, and its surgical margins. When MRS is used coupled with the DWI, it might provide a powerful non-invasive advanced tool that can arcuately characterise the tumour without further need for post-contrast series. Moreover, it can reduce the rate of unnecessary invasive biopsies.
Limitations
The current study had some limitations. First, it was a single-centre study with a small sample size. Second, the MRS technique is affected by technical factors such as lipid peaks overlapping with lactate peaks, or patient factors such as movement. Third, there might be potential selection bias. Finally, the study did not include normal cases.
Conclusions
MRS shows good diagnostic accuracy in predicting high-grade cervical cancer. Therefore, it might be considered a useful imaging technique in assessing the grade of cervical cancer to improve the planning of treatment. Furthermore, it can be adopted in clinical practice for better patient outcomes.