Introduction
Mediastinal masses encompass a wide range of malignant and benign pathologies. Cross-sectional imaging with contrast-enhanced computed tomography (CECT) is consi-dered the gold standard modality for the evaluation of these masses [1]. In most instances, they must be biopsied to narrow down the radiological differentials and to arrive at a definitive tissue diagnosis. Deep-seated mediastinal lesions are invariably precarious for performing percutaneous biopsy and are associated with multitude of complications ranging from pneumo/haemothorax, haemomediastinum, haemo-ptysis, and injury of major adjacent vascular structures/central airways, sporadically leading to death [2]. The technical intricacies of the percutaneous biopsy are compounded by the needle traversing through 4 layers of pleura and the extensive length of lung parenchyma, the dangerous site of the lesion surrounded by major viscera, and the cumulative time for the target needle placement, which in turn depends upon the operator’s expertise [3]. Technical innovations to minimize the chances of these complications such as use of thin-bore needles and fluoroscopy-guided endovascular needle biopsy have been described in the literature [4-6]. In this article, we would like to put forward a novel technique that we employed successfully in performing biopsies of 3 precarious mediastinal lesions.
Material and methods
Technique
We implemented an innovative technique in 3 patients who presented to us with mediastinal masses in close proximity to the superior vena cava. Preliminary CECT of the thorax was available for all of them. Careful evaluation of the lesion site and possible approaches were analysed. Probable angle of needle entry and required length of vascular sheath to reach the proximal margin of the lesion were also assessed. Using the Seldinger technique, a 6 Fr vascular sheath was placed, under USG and fluoroscopy guidance, into the right internal jugular vein. The angle of needle entry was made in such a way that the vascular sheath perpetuated the intended needle track. Under fluoroscopic guidance, the vascular sheath was approximately positioned adjacent to the proximal margin of the target, utilizing the bony landmarks derived from pre-procedural CECT. The vascular sheath was secured to the skin with adhesive dressings, and the patient was shifted to CT table in the same posture while maintaining sterile precautions. A check CT was performed initially to confirm the intended position of the vascular sheath (Figure 1B). The vascular sheath was adjusted accordingly to align the tip of the sheath just proximal to the target lesion. To perform the biopsy we used a Quick Core® biopsy needle set 20 G × 20 cm, 20 mm T (Cook Medical) in all 3 patients. The needle and sheath assembly is depicted in Figure 1D. Choice of a 20 G needle rather than 18 G was made based on the assumption that the lower the bore size of the needle, the fewer the complications. In the next step, we approximated the tips of the co-axial needle and the vascular sheath. Depending on the lesion location, the sheathco-axial needle unit was angled accordingly and the needle was advanced up to maximum of 10 mm in a single session. The needle position within the target lesion was confirmed under CT (Figure 1C). In addition, we also appreciated the absent blood return through the co-axial needle on removal of the inner stylet. Furthermore, a biopsy needle was introduced and multiple cores (maximum of three 20 mm cores in all 3 patients) were taken. In between needle exchanges we frequently flushed the vascular sheath with normal saline to prevent any inadvertent thromboembolism. Post procedure, the vascular sheath was removed and haemostasis was achieved by manual compression.
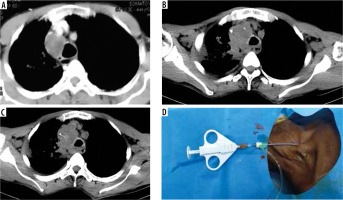
Figure 1
Illustration of computed tomography (CT)-guided trans superior vena caval biopsy in Case 1. A) Post-contrast CT image showing heterogeneously enhancing lesion in right paratracheal region causing near total compression of superior vena cava. B) 6 Fr vascular sheath in superior vena cava just proximal to the target lesion. C) Sample notch of 20 G × 20 cm, 20 mm T biopsy gun traversing through the target lesion. D) 6 Fr vascular sheath in right internal jugular vein. 19 G co-axial needle and 20 G × 20 cm, 20 mm T biopsy gun introduced co-axially through the vascular sheath
Case 1
A 65-year-old man presented with complaints of non-productive cough and hoarseness of voice for 2 months. CECT thorax revealed a heterogeneously enhancing mass lesion in the right paratracheal region extending inferiorly to the subcarinal and right hilar regions. The lesion was centrally located and seen in close vicinity of major vascular structures. Anteriorly, the lesion was causing near total compression of the superior vena cava and probable encasement of the right brachiocephalic trunk, ascending aorta, right pulmonary artery, trachea, and right and left main bronchi (Figure 1A-C). The critical location of the lesion made percutaneous biopsy tricky and dangerous in all the approachable routes. Anterior mediastinal or trans-sternal approach were ruled out due to lack of a safe window and the presence of arch vessels and the superior vena cava along the track. Similarly, lateral and posterior approaches were unfavourable because we would have to traverse a long distance of lung parenchyma, crossing 4 layers of pleura, which would increased the likelihood of complications.
Case 2
A 47-year-old woman presented with complaints of abdominal pain for 2 months. She was previously diagnosed as carcinoma cervix stage IIB and treated with definitive chemotherapy and radiotherapy. Further evaluation with PETCT was done, which revealed multiple FDG avid metastatic lesions in the vertebrae, and metastatic mediastinal and retroperitoneal lymph nodes. To move forward with a further treatment regimen, tissue diagnosis from one of the metastatic sites was essential. Of all the metastatic sites, the right paratracheal lymph node expressed the highest metabolic activity. Similarly to our first case, this lesion was also deep seated and perilous surrounded by major visceral structures, essentially ruling out all the possible percutaneous transthoracic approaches. Representative technical images are illustrated in Figure 2A-F.
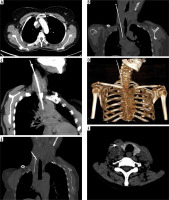
Figure 2
Representative images of same technique in Case 2. A) Axial section of contrast enhanced computed tomography (CT) depicting heterogeneously enhancing lesion in right paratracheal region with loss of fat plane between the lesion and superior vena cava. B) Needle-sheath assembly with sample notch of biopsy gun advanced into the right para tracheal lymph node through trans superior vena caval approach. C) Maximum intensity projection (MIP) image illustrating the exact needle placement within the right para tracheal node. D) Volume rendered image depicting the needle-sheath assembly. E) Oblique reconstruction image showing inadvertent puncture of co-axial needle through proximal part of vascular sheath. F) Axial CT image showing vascular sheath in right internal jugular vein and minimal hematoma surrounding the medial wall of right internal jugular vein due to inadvertent needle puncture
Case 3
A 35-year-old female came with complaints of back pain and difficulty in walking for 5 months. Initial imaging evaluation with MRI revealed an enhancing mass lesion in spinal cord at the D11-D12 level. Further on, PET-CT showed hypermetabolic non-necrotic and non-calcified mediastinal lymph nodes with right paratracheal lymph node harbouring the highest metabolic activity (Figure 3B). Tissue diagnosis was necessary to strategize the management plan. In a similar fashion to our last 2 cases, this lesion was also stationed in a tricky location, making the percutaneous transthoracic approaches less preferable. A needle-sheath assembly was positioned and the biopsy was performed as per the technique described above (Figure 3A-D).
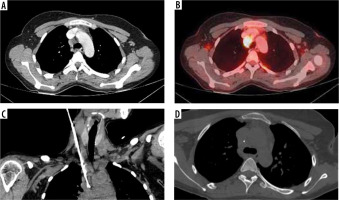
Figure 3
Procedural images of Case 3. A) Loss of fat plane between heterogeneously enhancing right paratracheal lesion and superior vena cava as evidenced in contrast-enhanced computed tomography (CT). B) Axial section of positron emission tomography CT depicting FDG avid right para tracheal lymph node with highest metabolic activity. C) Oblique reconstructed image showing the needle-sheath assembly. D) Biopsy needle traversing through the centre of the target lesion
In all our 3 cases, considering the possible significant number of complications associated with transthoracic approaches, our novel technique of CT-guided trans superior vena caval biopsy was successfully performed. Minimal neck haematoma occurred in our second case due to inadvertent puncture of the medial wall of the internal jugular vein during needle positioning (Figure 2E, F). An overall summary of the clinical presentation, location and size of the lesion, histopathological diagnosis, procedural time, and procedural complications is shown in Table 1.
Table 1
Clinical presentation, location and size of the lesion, histopathological diagnosis, procedure time, and complications of all 3 case scenarios
Discussion
In this new technique, we have employed both fluoroscopy and computed tomography guidance. In our initial experience with this technique, the significant advantages include the assessment of real time needle position, low radiation exposure, and very low risk of bleeding and pneumothorax. Although endobronchial ultrasound (EBUS)-guided biopsy of deep-seated mediastinal lesions has been described in the literature, it still harbours potential risks of haemoptysis due to the risk of bleeding into the central airways, and it is a much more invasive procedure [7]. A great advantage of our technique is that if bleeding occurs along the needle track, it tends to bleed into the vascular space rather than into extravascular spaces. To the best of our knowledge, this technique has not been described in the literature and is unique. We would like to highlight the significance of appropriate selection of cases for this technique. All 3 lesions described in this article were contiguous with superior vena cava and loss of fat planes between them. The angle of the needle entry into the internal jugular vein plays a vital role in enhancing the procedure progression. The mean procedural time for all 3 cases was 41 minutes. All 3 cases were performed by well-trained and experienced interventional radiologists. This technique warrants vigilant and precise needle placement to avoid any serious complications. It is important to emphasize that performing this novel technique of endovascular biopsy demands expertise and knowledge of the operator in both CT and fluoroscopic guidance. Future studies with large sample sizes are recommended to assess the overall safety of this technique. Modern hybrid consoles with combined CT and fluoroscopy are exemplary arenas for performing such innovative techniques.
Conclusions
As interventional radiologists, we encounter challenging referrals in our day-to-day practice. Such referrals warrant innovative thinking and expertise towards successful accomplishments. Through this article, we like to accentuate and enlighten our minds for future technical innovations in the interventional radiological fraternity.


