Introduction
An aneurysm is defined as a focal pathological dilatation greater than 50% of the normal diameter of a healthy segment of a blood vessel; the aorta is the largest artery in the human body and one of the most commonly affected by this condition [1,2]. The etiological factors that lead to the formation of aneurysms are diverse and include vasculitis, connected tissue disorders, infectious causes, or secondary to dissection, but the degenerative etiology associated with age and atherosclerosis is the most prevalent and well studied in the vascular surgery field and most commonly affects the infrarenal aorta [1,2]. The prevalence of abdominal aortic aneurysms (AAA) has been reported in the range from 0.5 up to 6% in male patients aged 65 years and older, although also a decline has been observed in some regions of Europe [3]; and the recognized risk factors include arterial hypertension, dyslipidemia, family history of AAA, smoking and male sex [2-4].
AAA are characterized as a progressive and life-threatening condition, and rupture is associated with elevated mortality; therefore, efforts to detect these arterial lesions in early stages are important to reduce aneurysmrelated mortality [2-5]. Nowadays, the European Society for Vascular Surgery in the most recent clinical practice guidelines recommends an ultrasound scan in men aged between 65 and 75 years with smoking history and AAA family history [2]. Once detected, these arterial lesions are monitored by ultrasound and computed tomographic angiography, and currently elective repair is recommended in men with AAA greater than 55 mm in diameter and in women greater than 50 mm [2,4]. Those with subtherapeutic diameters are defined as small AAA [2,6].
Histological analysis of the aneurysmal tissue revealed that it is characterized by transmural infiltration of inflammatory cells, mainly macrophages, depletion of vascular smooth muscle cells (SMCs) and degradation of arterial extracellular matrix (ECM) [5]. It remains unknown why some AAA show faster growth, but increased inflammatory activity in the aortic wall has been demonstrated [5,7,8].
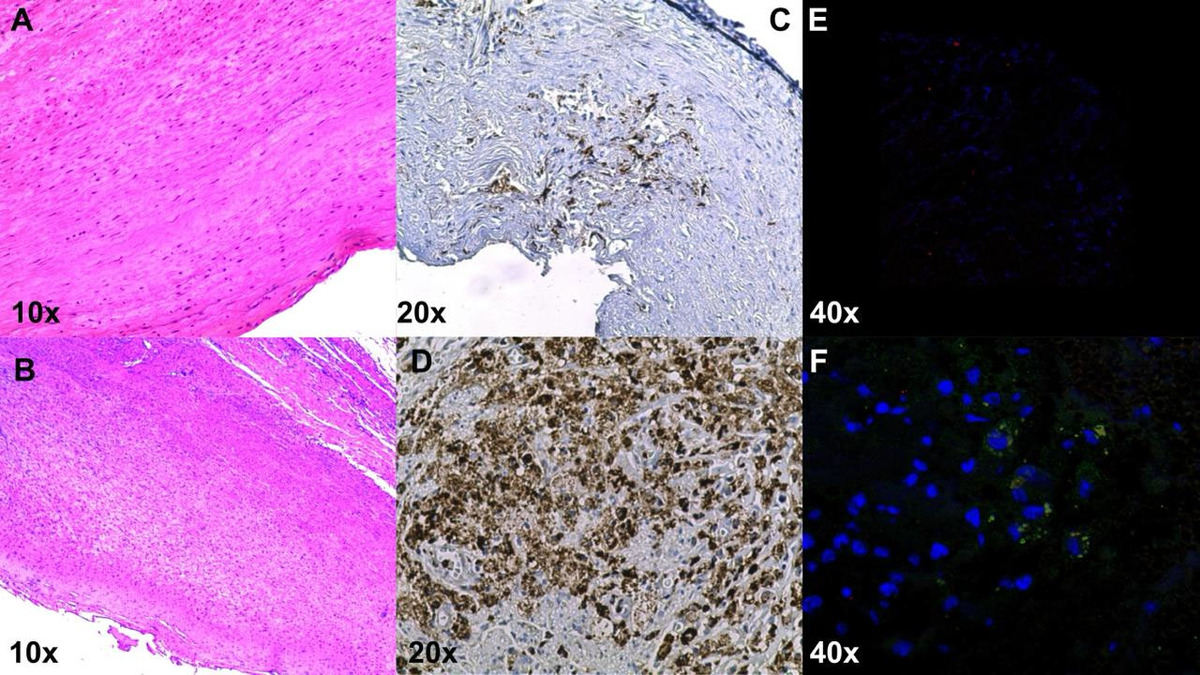
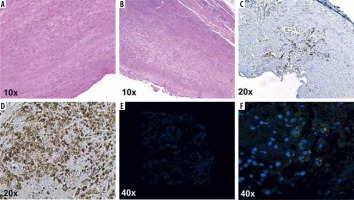
Monocyte chemoattractant protein-1 (MCP-1/CCL2) is a chemokine that plays a key role in migration and infiltration of monocytes/macrophages. In interaction with cytokines and cell adhesion molecules it mediates the trans-endothelial migration of monocytes to the subendothelial layer via cysteine-cysteine chemokine receptor-2 (CCR2). As a response to the chemotactic signal, monocytes upregulate the number of CCR2 receptors on their cell surface [9-11]. The presence of macrophages with the biomarker CD68 was demonstrated in aneurysmal tissue by immunohistochemistry and MCP-1 by immunofluorescence in one of our patients who underwent open AAA repair (Figures 1A-F).
Figure 1
Histological sections of the aorta with well-preserved tunica media stained with HE (A). Abdominal aortic aneurysm sections with marked dilatation and wall thinning, numerous lymphocytes, and plasma cells infiltrate adventitia in human aneurysm sections (B). Extensive subintimal fibrosis and inflammatory infiltrate, with extensive areas of macrophages (CD68) compared to normal aortic tissue (C, D). Double immunofluorescence staining of macrophages and CCLR in the intima of human aneurysm sections, CD68 (red); CCL2 (green); nucleus, DAPI (blue); in comparison with the normal aortic tissue (E, F)
The S-(1-(6-hydrazinylpyridin-3-yl)-2,5-dioxopyrrolidin-3-yl)-CKLFTGL peptide (HYNIC-CCR2-L), a specific C-C-chemokine receptor-2 ligand, can be easily labeled with technetium-99m (99mTc), considering its features such as optimal half-life, easy availability of the isotope, low cost and good stability [12]. Due to its biological recognition, it has been used for the detection of tumors [12], and the advantage of using HYNIC is its high labeling efficiency. The National Institute for Nuclear Research – Mexico has developed radiolabeled 99mTc-HYNIC-CCR2-L to assess the metabolic activity and progression of AAA. Recently, researchers reported detection of chemokine receptor 2 with positron emission tomography (PET) and its possible theragnostic value in animal models and humans [13]. We aimed to evaluate the expression of CCR2 specifically in patients with small AAA using single-photon emission computed tomography (SPECT) with the radiotracer 99mTc-HYNIC-CCR2-L (Figures 2A, B).
Material and methods
The pilot study was designed to evaluate patients with small asymptomatic AAA with nuclear imaging. The equipment used was a Symbia T2 (Siemens, Germany) with radiolabeled 99mTc-HYNIC-CCR2-L developed in the National Institute of Nuclear Research – Mexico. The SPECT uptake and activity were assessed and counted based on the ‘region of interest’ (ROI), and nonparametric statistics were employed to analyze and compare the aneurysm site, left ventricle (Control 1) and aortic regions with nondiseased aortic tissue (Control 2) in the same individual. Statistical analysis was performed with GraphPad Prism 9.0 software. The study protocol was approved by the Institutional Review Board at the National Institute of Medical Sciences in Mexico City with the reference number 4099.
Results
The 3 patients were male (100%) (mean age 81 years, standard deviation [SD] 11, mean AAA maximum diameter of 40 mm SD 3 mm): one with a past medical history of arterial hypertension, one with type 2 diabetes mellitus and two of them with dyslipidemia. The three were former smokers without a known family history of aneurysmal disease in the aorta. Patient one had a maximum AAA diameter of 43 mm and ROI of 51,145 (Figures 3A-C); patient two had a maximum AAA diameter of 36 mm and ROI of 33,834 (Figures 4A-C); and patient three had a maximum AAA diameter of 43 mm and ROI of 28,370 (Figures 5A-C).
Figure 3
Patient 1, 82-year-old male patient with a small abdominal aortic aneurysms measuring 43 mm in maximum diameter in axial view (A). The coronal projection demonstrates the location in relation to intraabdominal organs (B) and the SPECT analysis shows the ROI value of 51,145 (C)
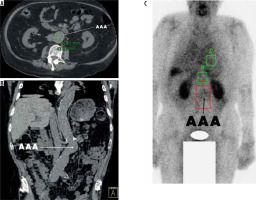
Figure 4
Patient 2, 92-year-old male patient with a small abdominal aortic aneurysms measuring 36 mm in maximum diameter in axial view (A). The coronal projection demonstrates the location in relation to the intraabdominal organs (B) and the SPECT analysis shows the ROI value of 33,834 (C)
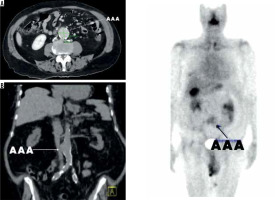
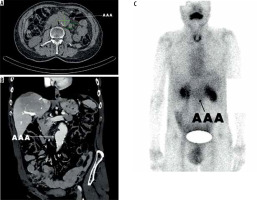
Figure 5
Patient 3, 70-year-old male patient with abdominal aortic aneurysms measuring 43 mm in diameter in axial view (A). The coronal projection demonstrates the location in relation to intraabdominal organs (B) and the SPECT analysis shows the ROI value of 28,370 (C)
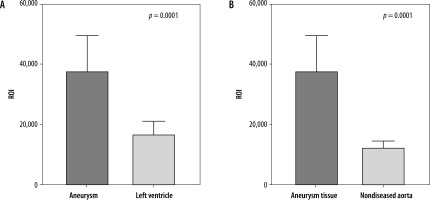
The patients tolerated the nuclear studies well, and images were obtained at one, two and four hours. The ROI mean value of the aneurysm site was 37,783 (SD 11,890), compared to 16,779 (SD 4397) for the left ventricle (Control 1) (p-value = 0.0001); ROI for the healthy aortic tissue (Control 2) was significantly lower, 12,520 (SD 2141) (p-value = 0.0001) (Figures 6A-C).
Discussion
The growth of AAA seems to be potentially driven by several pathophysiological mechanisms associated with inflammation, proteolysis, angiogenesis and ECM degradation [2-15]. Macrophages are key components of inflammatory processes and are mainly divided into two subtypes (M1 and M2) [16]. Studies have shown that activated M1 macrophages secrete a variety of proteases, such as matrix metalloproteinases (MMPs) and cathepsin, which are involved in ECM degradation in the vascular wall, which promotes remodeling of the abdominal aortic wall [16-18]. M2 macrophages are also able to release interleukin 13 and chemokines such as MCP-1/CCL2 [18-20]. This knowledge led us to investigate this chemokine and its receptor.
The recruited monocytes express MCP-1 by themselves, which accelerates the further influx of mononuclear cells. Once within the neointima, the monocytes differentiate into macrophages, which express MMPs, and foam cells induce dissolution of SMCs and collagen, which causes the plaque cap to become thin and susceptible to rupture. Studies have suggested that the MCP-1/CCR2 axis might be involved in the pathogenesis, progression, and morphologic and structural stability of AAA [14,21].
Presently, abdominal duplex ultrasound (DUS) is the first line imaging tool for the detection and surveillance of small AAA, with a high sensitivity and specificity [2]. The diameter of the aneurysm is nowadays the most important factor to consider for the risk of rupture, and this has been estimated below 1% at one year for 50 mm diameter AAA, at four years for 40 mm diameter AAA, and at eight years for 30 mm diameter AAA [2,4,6]. Thus, the establishment of predictors is crucial for prevention of death due to the high AAA mortality rate, which can reach up to 90% when rupture occurs [2].
Molecular imaging has shown application in murine models and potential for well-designed human clinical research studies [22-26]. Several authors have reported that there is increased uptake of fluorine-18-fluorodeoxyglucose-6-phosphate (18F-FDG) by PET in the aneurysmal wall, which implies an inflammatory process at that level, and that they have increased metabolic activity associated with a high density of inflammatory cells in the wall that leads to its growth and greater risk of rupture [26]. Recently studies have also been performed with ultrasmall superparamagnetic particles of iron oxide (USPIO) that detect cellular inflammation on magnetic resonance imaging (MRI). They were found to predict the rate of aneurysm expansion as well as the future risk of AAA rupture or need for repair [27].
We opted to use SPECT in this particular early AAA stage, because of its relatively low cost and accessibility. The most commonly used radiopharmaceuticals today are those containing technetium radionuclides (99mTc) because of their favorable nuclear properties for imaging and therapy in the treatment of various diseases, including cardiovascular [12,28]. The National Institute for Nuclear Research – Mexico has published a study in which they designed and evaluated an inhibitor of the HYNIC peptide conjugated with 99mTc and a ligand (PD-L1) [99mTc]Tc-iPD-L1), which demonstrated its ability to differentiate tumor lesions expressing PD-L1 protein from those that do not. It can be considered a potentially useful tool for immunotherapy in cancer patients [12]. Therefore, 99mTc-HYNIC-CCR2-L may be an efficient molecular radiotracer because of the intense radiotracer uptake along the AAA wall, while little uptake signal was observed in healthy tissue. It provides objective imaging reference indicators in the prognosis and possible risk of rupture of AAA. Molecular imaging techniques such as SPECT and PET have the advantage of being non-invasive, in vivo, with high sensitivity, adequate spatial resolution, and image acquisition times in minutes. Unfortunately, PET is not widely available in some areas. Although a variety of tracers have been used in AAA prognosis in clinical and animal models, there is an unmet clinical need to develop highly specific and sensitive tracers to identify, diagnose and evaluate cases of progression of AAA that are prone to expand and/or rupture. In the future, the development of radiotracers such as 99mTc-HYNIC-CCR2-L may prevent fatal outcomes such as death in these patients, as well as reducing the costs that arise from emergency surgery due to a ruptured aneurysm.
Study limitations
We recognize that the study has limitations: it was a pilot study with a small number of patients, with similar characteristics, leading to possible selection bias. However, these initial findings demonstrate the feasibility of studying small AAA longitudinally to determine whether there is a relationship between lesion expansion and possible risk of rupture. These observations encourage future and more precise research in this particular area.
Conclusions
SPECT revealed significant differences in CCR2 expression in the AAA site compared to the left ventricle and nondiseased aortic segments. Well-designed longitudinal studies with nuclear imaging modalities may assist in the molecular characterization of aneurysmal progression and rupture prediction. The attractive features offered by SPECT are that it is more accessible and less expensive than PET, which makes it more readily available. The SPECT radiotracer can be specifically captured by over-expressed receptor proteins in various tissues, providing functional and metabolic information. The SPECT study is fast, and once completed, the patient can return to normal activities.