Introduction
Intrauterine growth restriction (IUGR) is the second most common cause of perinatal mortality. Its prevalence among all newborns is reported to be 5-10% [1,2]. It is strongly associated with adverse pregnancy outcomes and an increased likelihood of abnormal long-term neurodevelopmental complications including cerebral palsy, learning disabilities, and behavioural problems [3,4].
Post-mortem examination of IUGR foetuses shows loss of both grey and white matter substructures, including reduced cell number, disorganised cortical structure, and unmyelinated axons with thinner myelin coverage [5,6]. Detection of neonates who will develop neurodevelopmental impairment in the future is challenging. Therefore, neurostructural imaging findings such as changes in gyrification, cortical morphometry, connectivity, and metabolism are suggested to have prognostic value [1,6-11].Not only early severe IUGR foetuses will develop impaired neurocognition, but also foetuses with milder, late-onset forms – which are the majority of IUGR cases – can be affected [1].
The adverse neurodevelopmental outcome in IUGR foetuses is mainly the result of placental insufficiency and chronic hypoxia [2,4]. In a clinical setting, to determining the severity of utero-placental insufficiency and hypoxia, these high-risk pregnancies are monitored with various methods including non-stress test, sonographic biophysical profile, and Doppler ultrasound [2,5,6]. These assessments should be done to determine the delivery time and protect foetuses from the risks of hypoxia.
Foetal brain magnetic resonance imaging (MRI) is used to provide distinctive information about the structural and functional status of foetuses [12]. Restricted brain growth, delayed brain gyration, or focal ischaemic/haemorrhagic lesions could be investigated by conventional MRI; however, identifying diffuse ischaemic changes or earlier and subtle injuries remains challenging [4]. Microstructural abnormalities are also not detectable by conventional MR sequences but could be identified by diffusion-weighted MR imaging (DWI) with altered apparent diffusion coefficient (ADC) values [2,4].
Because there are relatively few studies that have investigated the role of DWI in the IUGR foetal brain, this study aims to evaluate the ADC value differences in various brain regions between IUGR foetuses and normal controls using DW imaging.
Material and methods
Patient population
This prospective study was performed in a university teaching hospital between April 2017 and February 2019. The study was approved by the institutional review board (IRB) of Tehran University of Medical Sciences. Written informed consent was obtained from all women who participated in the study.
Gestational age in all foetuses was established based on the first-trimester crown-rump length (CRL) measurement. IUGR was defined as a foetal estimated weight less than the10th percentile for gestational age according to the reference data and based on ultrasound measurement [13].
The control group was selected from non-IUGR foetuses with similar gestational age, referred for foetal MRI for suspected ventriculomegaly (n = 2), duodenal atresia (n = 2), oesophageal atresia (n = 1), mega cisterna magna (n = 2), posterior urethral valve (n = 1), macrosomia (n = 1), syndactyly (n = 1), hemivertebra (n = 2), cleft lip and palate (n = 1), pulmonary cyst (n = 1), and those with normal foetal MRI and normal outcome (n = 4).
Exclusion criteria were as follows: multifoetal pregnancy, history of congenital infection, abnormal brain biometry, chromosomal anomalies, or suspected genetic syndrome. Additionally, any structural or brain abnormalities that were detected on ultrasound or conventional MRI sequences and non-diagnostic DWI sequences with blurred ADC maps (due to motion artefact degradation) were also excluded.
Four cases in the IUGR group and two of the control group were excluded according to the degradation of DW image by foetal motion. We also lost three IUGR cases (due to emergent caesarean delivery: two with severe hypoxia, who delivered their babies before MRI examination appointment, and one due to premature rupture of membranes). Eventually, a total of 38 foetuses with IUGR and 18 from the control group with similar gestational weeks (28-38 weeks) were included.
We recorded the foetal gestational age, biometry, and weight at the time of MRI examination as well as birth weight and pregnancy outcome following delivery.
Doppler ultrasound examinations
All IUGR foetuses underwent Doppler examination by a trans-abdominal convex array transducer with 2-6 MHz frequency (Affiniti 50, General imaging configuration, Philips ultrasound machine, USA) in the same week of MRI examination. Foetal umbilical artery (UA) and middle cerebral artery (MCA) pulsatility index (PI) and the mean bilateral uterine artery (UtA) PI were measured, and values greater than the 95th percentile were considered abnormal. The cerebroplacental ratio was also calculated, and values less than the 5th percentile were considered abnormal according to the reference chart [14]. All Doppler studies were performed in accordance with the International Society of Ultrasound in Obstetrics and Gynaecology (ISUOG) practice guidelines [15]. IUGR cases were subdivided into two study groups: IUGR with severity signs (group A) defined by birth weight less than the third centile and/or abnormal UA or UtA PI and/or abnormal Cerebroplacental ratio (< 5th centile), and group B without any of these criteria.
Magnetic resonance imaging protocol
Foetal MRI was performed using a 3T MR unit General Electric system (GE Healthcare, discovery 750 GEM), with a 16-channel phased-array coil following four hours of maternal fasting. The IUGR group underwent MRI examination within the same week of diagnosis. No foetal or maternal sedation was used during the MR study, and patients were positioned in the left lateral position on the MR scanner table. As part of the foetal MR examination, conventional sequences for all women included 4 mm single-shot fast spin-echo T2-weighted sequences in three orthogonal planes and axial fast multiplanar spoiled gradient-recalled acquisition in the steady-state T1-weighted sequences in an axial plane. Only T1-weighted sequences were performed with breath-holding.
Foetal brain DWI [b value = 0-700 s/mm2 in three orthogonal directions] was performed using single-shot spin-echo-planar imaging (EPI) in an axial plane without breath-holding [TR, 3000 ms; TE, min; FOV, 320-400 mm; matrix, 128 × 128; section thickness, 4-5 mm; space, 1 mm; and band-width, 250 kHz; acquisition time, 20 s]. If any motion artefacts or any distortion were detected, the sequence was repeated until a satisfactory image was obtained; however, we excluded four cases from the IUGR group and two cases from the control group due to the poor quality of the DWI images. ADC mapping was performed on a picture archiving and communication system (PACS). The total mean examination time was 30 ± 5 min per patient.
Magnetic resonance imaging analysis
Conventional sequences
Brain morphology and signal were assessed by a faculty member radiologist (B.M.: the first author) with five years’ experience in foetal imaging and foetal brain MRI. Brain biometry (biparietal diameter [BPD] and head circumference [HC]) was assessed according to the reference chart [13]. The reader was aware of the preliminary diagnosis of IUGR but not the subtype.
Diffusion-weighted magnetic resonance imaging sequences
Multiple circular surfaces, 30-60 mm regions of interest (ROI), were drawn in different brain regions and plotted on ADC maps by the same radiologist. ROIs were drawn in the frontal white matter (FWM), occipital white matter (OWM), centrum semi-ovale (CSO), thalami, cerebellar hemispheres (CH), and Pons according to the criteria described in previous studies [4,16]. Also, we evaluated other regions according to the following criteria (Figure 1):
temporal lobe white matter (TWM): in the middle of the white matter of the temporal lobe on the axial slice at the level of the midbrain (ROI: 30 to 60 mm2),
hippocampal cortex (HC): at the same level described above, in the grey matter of the hippocampus (ROI: 5-15 mm2),
Rolandic cortex (RC): at the uppermost cut on the axial slice on the Rolandic cortex (ROI: 5 mm2),
caudate nucleus (CN): in the middle of each caudate nucleus on the axial slice at the level of frontal horn and cavum septum (ROI: 5 mm2).
Figure 1
Localisation of the regions of interest (ROIs) within all brain regions. A) Frontal white matter and occipital white matter, B) centrum semi-ovale, C) Rolandic cortex, D) thalami and caudate nucleus, E) temporal lobe white matter and hippocampal cortex and f) pons and cerebellar hemispheres
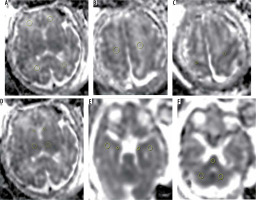
ROI size varied according to the gestational age and size of the foetal brain region of interest. Adjacent structures such as CSF spaces were avoided. All measurements were performed on a PACS workstation. The automatically measured average signal intensity was recorded as the ADC value. The average of ADC values (10-30 mm2/s) in each hemisphere were compared within each group of IUGR cases and with the control group.
Follow-up of Intrauterine growth restriction cases
All foetuses had at least one scan within one week of delivery. Follow-up postnatal data were prospectively collected throughout the study with a questionnaire by phone.
Statistical analysis
Data were analysed using SPSS software, version 22 (IBM Corp., Armonk, NY, USA). Descriptive statistics were assessed. In the data analysis, first the normal distribution of quantitative data was investigated using the Kolmogorov-Smirnov test of a K-S sample. Regarding the distribution of data (normal and abnormal), Fisher’s, chi-square, and T-tests were used at the significance level of less than 0.05. ADC values are also presented as mean with standard deviation.
Results
Population study characteristics
A total of 38 foetuses with IUGR and 18 of the control group with nearly similar gestational age (approximately 34 weeks) were investigated. IUGR foetuses included 23 with clinical severity signs (group A) and 15 without clinical severity signs (group B).
There was no significant difference between IUGR foetuses and the control group regarding maternal age, body mass index (BMI), and foetal gestational age (29.89 ± 5.44 years in the IUGR group vs. 29.81 ± 3.22 years in the control group; 29.59 ± 5.16 kg/m2 in the IUGR group vs. 29.4 ± 4.99 kg/m2 in the control group, and 34 weeks + 2 days in the IUGR group vs. 34 weeks in the control group respectively). Foetal weight was lower in IUGR cases compared to the control group (1787.3 ± 506.7 γ vs. 2053.2 ± 734.96 g, respectively), but the difference was not significant (p = 0.1). There was no significant difference in foetal sex between two groups of IUGR. However, the number of male foetuses was significantly higher in the control than in the IUGR group (77% vs. 52%, p = 0.04). Considering the history of preeclampsia, there was a significant difference between the IUGR and control group and between IUGR subtypes (13.2% in IUGR foetuses vs. 0% in the control group, and 17.4% in IUGR A vs. 6.7% in IUGR B; p = 0.002 and 0.04, respectively). HC < 5% was more common in IUGR A compared to IUGR B (56.5% vs. 13.3%, p < 0.0001) (Table 1).
Table 1
Maternal and foetal characteristics of the study groups and perinatal outcome
Doppler study results
In IUGR group A compared to the other subtype, the UA PI and UtA PI were higher and MCA PI was lower, but the differences were not significant (Table 2). Ten (43%) foetuses in IUGR group A had UA PI > 95th centile, four (17%) foetuses had MCA PI < 5th centile and abnormal cerebroplacental ratio (brain sparing), and five (21%) had UtA PI > 95th centile. There were no foetuses with absent or reversed diastolic flow in the umbilical artery or abnormal flow in ductus venosus in group A (no IUGR cases had severe hypoxia).
Magnetic resonance imaging examination
Conventional sequences
Brain white matter and grey matter signal and morphology were normal in all foetuses and no haemorrhagic event like intraventricular or germinal matrix haemorrhage was found in any cases.
Diffusion-weighted magnetic resonance imaging sequences
In all o the lowest ADC values were detected in the pons, followed by RC, HC, cerebellum, and thalami and the highest ADC values were detected in FWM, OWM, and TWM.
In a comparison between IUGR foetuses and the normal group, the ADC values were significantly lower in CH (1.239 ± 0.164 vs. 1.280 ± 0.188 × 10-3 mm2/s, p = 0.045), thalami (1.205 ± 0.170 vs. 1.275 ± 0.158 × 10-3 mm2/s, p = 0.031) and CN (1.319 ± 0.173 vs. 1.394 ± 0.166 × 10-3 mm2/s, p = 0.04) (Figure 2). However, there was no significant difference between them in ADC values of other brain regions. A comparison of ADC values between the two groups is outlined in (Table 3). Among IUGR subtypes, ADC values were not significantly different in any brain region but were slightly lower in HC (1.250 vs. 1.296 x10-3 mm2/sec), thalami (1.182 vs. 1.239 × 10-3 mm2/s), cerebellum (1.228 vs. 1.257 × 10-3 mm2/s), RC (1.180 vs. 1.226 × 10-3 mm2/s), and CN (1.300 vs. 1.348 × 10-3 mm2/s) and were slightly higher in other areas in group A (Table 3.).
Figure 2
Differences between IUGR foetuses and the control group. The polynomial contrast analysis figure demonstrates the differences between IUGR and non-IUGR foetuses. The bars depict the mean ± SD within the groups. Asterisks indicate p < 0.05
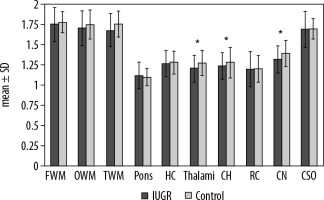
Table 3
ADC values in IUGR subtypes, IUGR, and control group in different brain regions
Postnatal follow-up
In the postnatal follow-up, the foetal birth weight was significantly lower in IUGR compared to the control group (2365.5 ± 529.3 γ vs. 3018.8 ± 664.8 g, p = 0.01). Birth weight was also significantly different between IUGR groups A and B (2198.5 ± 558.8 γ vs. 2604.2 ± 386.84 g, p = 0.001). There was no significant difference between the two groups and IUGR subtypes regarding gestational age at birth and the rate of NICU admission. Caesarean delivery was more common in the IUGR group (83% vs. 50% in the control group, p = 0.006). Only one death (stillbirth) in subtype A was reported (Table 1).
Discussion
DWI is based on proton diffusive motion, and one of its properties is the ability to identify acute or chronic pathologic changes that are not detectable on conventional MR sequences [17]. However, it is still challenging to perform foetal brain DWI due to the motion of both the mother and the foetus [18].
Myelination, ischaemia, and infection are the main processes that decrease ADC values [2]. The DWI sensitivity to detect acute hypoxic-ischaemic events is dependent on its severity and duration, the extent of the affected brain, and the time point of foetal MRI. For example, subtle damage to the brain has no effect on ADC values [17]. Additionally, milder forms of hypoperfusion usually affect periventricular white matter in the premature brain and result in periventricular leukomalacia (PVL) as the most common pathologic finding. In severe hypoperfusion, the most metabolically active brain regions in the immature brain (≤ 36 weeks) include deep grey matter (the thalami and the brainstem), whereas in term infants the vulnerable areas are different and include lateral thalami, hippocampi, and sensorimotor cortex [19,20]. Evidence of mixed acute and chronic hypoxic injuries in histopathological examination of two foetal brains with severe IUGR has been shown by Arthurs et al. [4].
Apart from hypoxia, abnormal maturation is the other proposed mechanism that decreases the ADC values of white matter in IUGR cases. Normal ADC values of cerebral white matter increase gradually up to 30 weeks (possibly related to maximal cellular migration at this time) due to the maturation of brain parenchyma, and subsequently decrease after 30 weeks of gestation. Furthermore, the decline of ADC values in the occipital regions occurs more rapidly after 30 weeks in comparison with frontal lobes due to earlier myelination. Also, brain stem, CH, and thalami ADC values show a linear negative correlation with foetal age [4,16,21]. However, not all studies suggest this pattern of ADC changes with advancing gestational age [22-24].
Our results indicated that although all cases showed normal brain signals on conventional MRI sequences, IUGR foetuses demonstrated significantly lower ADC values than normal controls in CH, thalami, and CN. The decline in ADC values in thalami and CN was in accordance with the results of previous studies on ADC values of the foetal brain in IUGR cases. However, in contrast to the previous studies, we found no significant difference between ADC values of FWM or OWM in IUGR foetuses and the control group [2,4]. Considering that the average gestational age of our cases in both groups was about 34 weeks, the decline in ADC value of thalami and CN are consistent with hypoxia pattern in the immature brain, but RC (or somatosensory cortex) and HC (which, based on the data of previous research, are affected in hypoxic injuries of term infants) showed no significant change in ADC values. However, these findings might also be due to the effects of cerebral blood flow redistribution that alters the normal maturation and myelination.
In contrast to the results of previous research, which insisted on the cerebellum as the most resistant region to hypotension/ hypercarbia [2,3,25], the ADC values in CH were relatively low in our study. However, various studies show different findings, and reduced cerebellar area and volume in IUGR foetuses have been shown in some studies [5,6,10,11]. The discrepancy between results may be due to the heterogeneity of the IUGR population. Also, based on our results, pons had the lowest ADC value among all groups, and white matter generally had the highest ADC values, which are in agreement with the findings of previous studies [2,4,26,27].
Our DWI findings showed non-significant low ADC values in CN, HC, RC, thalami, and CH in IUGR foetus group A compared to group B. Previous studies indicate that infants with altered umbilical artery Doppler demonstrate significantly poorer motor and cognitive outcomes at around two years of age. Furthermore, decreased MCA PI (brain sparing effect) is associated with worsening hypoxaemia as well as increased incidence of foetal brain injury with subsequently neurological deficits at the age of two years [6,28,29]. However, it should be noted that the foetal brain is not spared from injury at any point of time, and brain sparing is a misnomer term. On the other hand, some previous studies suggest that late-onset IUGR foetuses with normal brain Doppler are not really “constitutionally” small, but possibly suffer a form of restriction [1,30,31]. The reason that we did not find a significant difference in ADC values of IUGR subtypes might be that Doppler changes were not severe and none of our IUGR cases had severe hypoxaemia-acidosis (no cases with absent or reverse diastolic flow in UA or abnormal flow in DV). Another reason may be related to our relatively small sample size. Also, it can also be related to brain autoregulation as it is trying to protect itself from hypoxia, which results in ADC changes to remain only at a microstructural level.
We had no IUGR cases with IVH even in foetuses with gestational age more than 34 weeks. There is controversy about the correlation between IVH and IUGR in the literature. Several studies considered IUGR as a risk factor for IVH, and other studies suggested a protective role for IUGR. It has been shown that the IVH rate is significantly lower in IUGR cases born ≤ 28 weeks but is more common in late IUGR (born ≥ 34 weeks) [6,32,33].
This study has some limitations. First, the sample size was limited, especially to find a significant difference in ADC values between IUGR groups. Second, we had no IUGR cases with severe hypoxia, and we lost two cases with severe vascular impairment due to emergent caesarean delivery. The inclusion of cases with severe abnormal Doppler indices might result in a statistically significant difference among the two IUGR groups. Third, we manually placed all ROIs, and that could be subject to inter/intra-observer variability.
Conclusions
In our study ADC values of some brain areas (thalami, CN, and CH) were significantly lower in IUGR foetuses in comparison with the control group. The ADC values were not significantly different between IUGR subtypes. The mean gestational age of our IUGR foetuses was about 34 weeks, and we detected nearly immature brain hypoxic involvement pattern without significant injuries to RC and HC. Further research with larger sample sizes is needed to evaluate the effect of hypoxia on brain involvement pattern based on ADC values on immature and mature foetal brain (≥ 36 weeks).


