Introduction
Intracranial dural arteriovenous fistula (dAVF) represents 10-15% of all intracranial vascular malformations [1-4]. Their aetiology is not fully understood; they are suspected to arise secondarily to inflammation or injury involving dural sinus. Fistulas usually occur in the area of the transverse and sigmoid sinus, and the cavernous sinus.
Fistulas can remain asymptomatic until a patient reaches middle age or older. Patients may report symptoms from mild, such as headache, dizziness, and tinnitus, to serious neurological disorders, often resulting from bleeding. Symptoms are closely related to the type of outflow from the fistula and its location [4].
There are different classification systems, including the one proposed by Borden, subsequently extended by Cognard, based on the assessment of the risk of bleeding [5,6]. Retrograde flow in the ectatic cortical vein is thought to be associated with the highest mortality and morbidity. These characteristics render prompt treatment: endovascular embolisation, surgical resection, or radiosurgery. Often, effective treatment is a combination thereof [4].
There are mixed reports on the incidence of intracranial haemorrhage in patients with dural arteriovenous fistulas. We tried to further facilitate risk assessment by implementing additional measurable angiographic characteristics of the fistula. Elaborating on the previous experiences and literature, we evaluated whether the number of outflows or the outflow diameter might add to risk assessment.
Material and methods
We retrospectively reviewed our patient database from January 2006 and December 2016 and identified 40 cases of dural arteriovenous fistulas. Patients with carotid-cavernous fistulas were excluded from the study, which ultimately yielded 25 patients with 28 dural arteriovenous fistulas. All patients were diagnosed and treated using a GE Innova 4100 Angiograph (GE Healthcare, Chicago, IL, USA).
Patient demographics and angiographic data were collected from the records. Angioarchitectural data included location, feeding arteries, number of outflows, biggest outflow diameter, Cognard classification type, treatment method, number of treatment sessions, volume of contrast media used, dose received by the patient, amount of time needed to treat, and outcome on DSA. The angiographic data were independently reviewed and evaluated by two experienced interventional neuroradiologists.
Patients in our study were divided into two groups: 1) with intracranial haemorrhage and 2) without. The diagnosis of intracranial bleeding was confirmed on a CT scan. Bleeding was attributed to dural arteriovenous fistulas in all patients.
Embolisation procedures were performed under general anaesthesia. Patients were given either Ultravist 300 (Bayer AG, Leverkusen, Germany) (before 2012) or Visipaque 320 (GE Healthcare, Chicago, IL, USA) contrast material (after 2012). Diagnostic cerebral arterial angiography was performed, including at least four vessels, through transfemoral access. A guide catheter was navigated into the appropriate parent vessel, and then a compatible microcatheter with a microwire were advanced selectively into the branches as close to the fistula as possible, allowing an adequate distance for reflux of the embolic agent. Embolisation of the fistulas was performed with a mixture of Histoacryl with lipiodol (2 : 1) and/or ONYX-18. The end point of the embolisation was the angiographic obliteration of the fistula.
Statistical analysis was performed using Statistica software (StatSoft, Inc., Tulsa, OK, USA). Descriptive statistics of all variables were calculated. Normally distributed quantitative variables were compared using Student’s t-test between patients with and without intracranial haemorrhage; non-normally distributed categorical variables were compared using the U-Mann Whitney test. Correlations between all variables were calculated using the Spearman Correlation Coefficient: patient demographics and angiographic data-included location, feeding arteries, number of outflows, biggest outflow diameter, Cognard classification type, treatment method, number of treatment sessions, volume of contrast media used, dose received by the patient, amount of time needed to treat, and outcome on DSA. The statistical level of significance was set at p = 0.05.
Results
Fourteen (56%) patients presented with intracerebral haemorrhage, headache was noted in five patients (20%), hypoesthesia in two patients (8%), hemiparesis in two patients (8%), blurred vision in one patient (4%), and epilepsy in one patient (4%). Seven patients (28%) had fistulas diagnosed during cerebral aneurysm work-up. Multiple dural fistulas occurred in three patients (12%). In five cases there was association with head trauma (20%).
Most fistulas drained into the transverse-sigmoid sinus (14 fistulas – 50%), six (21.4%) into the straight sinus, five (17.8%) into the superior sagittal sinus, and one each (3.6%) into the ethmoidal, cavernous, and foramen magnum (Table 1).
Table 1
Angiographic characteristics of fistulas and differences between non-haemorrhagic and haemorrhagic groups. Statistical significance set at p < 0.05
Spearman’s rank correlation coefficient revealed several statistically significant correlations. There was a strong association between Cognard classification type and time needed to treat a fistula, expressed either in minutes or number of sessions (r = 0.59, p < 0.05 and r = 0.51, p < 0.05; respectively), unsurprisingly affecting also the volume of contrast used (r = 0.77, p < 0.05). It was more challenging to close a higher Cognard classification type fistula, although the coefficient did not reach statistical significance (r = –0.33, p > 0.05), especially those located infratentorially (r = 0.53, p < 0.05) and on the right side (r = 0.66, p < 0.05).
Bleeding was associated with poorer clinical outcome as expressed by modified Rankin Scale score (r = 0.48, p < 0.05). Older patients tended to absorb significantly higher doses (r = 0.58, p < 0.05), which was associated with the time of the procedure (r = 0.67, p < 0.05)
Cognard classifications of all fistulas as well as other angiographic characteristics are presented in Table 1. We found no statistically significant differences between the non-haemorrhagic group and the haemorrhagic group regarding the age and sex of the patients (p = 0.106 and p = 0.54, respectively), location of the fistula (overall p = 0.86), number of outflows (p = 0.459), greatest outflow diameter (p = 0.298), Cognard classification type (overall p = 0.118), or procedure-related time and resources.
Clinical evaluation at follow-up of all patients was according to modified Rankin Scale (mRS). Fourteen (56%) patients were asymptomatic (score 0), six (24%) had non-significant or slight disability, maintaining independency, four (16%) had moderate disability, and two (8%) died, one in the course of intracerebral haemorrhage, and one due to other sustained injuries. Three (12%) patients were lost to follow-up. Clinical outcome on follow-up was significantly better for patients with no haemorrhage (p = 0.038).
Overall, we achieved a 70% angiographic occlusion rate (via arterial feeders). We did not consider dAVFs downstaged to type I in the course of treatment. There were no reported embolisation-related complications.
Discussion
Malik et al. [7] were among the first to identify cortical venous drainage as the main risk factor for haemorrhagic presentation of dural arteriovenous fistulas. Reported rates of haemorrhage and neurological symptoms not directly related to haemorrhage in patients with cortical venous drainage vary due to relatively small study groups. Intracranial haemorrhage and neurological symptoms are reported to be 4.5-35% and 7.2-30% annually, respectively [1,8-10].
More recently, Li et al. [11] evaluated clinical and angiographic characteristics of dAVFs associated with haemorrhagic presentation in a larger and more homogenous cohort of 236 patients. They found additional associations between intracerebral bleeding and tobacco and alcohol use, which we believe most of us intuitively advise our patients against. They confirmed the risk of all the angiographic patterns reported before.
We ventured to further aid in assessing the risk of haemorrhage by counting the number of cortical venous outflows and measuring the degree of venous ectasia in all fistulas. We believed there would be a direct association of at least outflow diameter. Statistical analysis failed to recognise this association. Actually, mean outflow diameter for the haemorrhagic group was smaller than for the non-haemorrhagic group (3.79 vs. 5.83 mm, respectively; p > 0.05). However, the relationship might be reversed in a larger cohort in long-term observation. Li et al. [11] looked at this problem from another perspective, evaluating multiple arterial feeders as a potential risk factor of cerebral haemorrhage, and found the opposite (82.8% vs. 67.9%, p = 0.016; for non-haemorrhagic and haemorrhagic presentation, respectively).
Contrary to many reports on high association of cortical venous ectasia with haemorrhage, in our study 63% of Cognard type IV fistulas had a non-haemorrhagic presentation as compared to 20% of those classified as type I. Overall, intracerebral haemorrhage was diagnosed in eight type I fistulas (which account for 30.7% of all fistulas) but only in four (15.2%) type IV fistulas. We believe the main reason is the inhomogeneity of our study group. One of the patients was diagnosed with a fistula in the course of diagnostic work-up of intracerebral haemorrhage after a severe motor vehicle accident, which was the direct cause of bleeding. On angiography, it was classified as type I, although it was of very high flow. We do not know if the patient developed the fistula after a head trauma and subsequently developed a substantial haemorrhage, or if the trauma led to disruption of a pre-existing fistula. The fistula was successfully closed with Onyx-18, but the patient died of other sustained injuries.
Post-traumatic dural arteriovenous fistulas represent a small proportion of all dural fistulas in scientific reports, mostly cases with no (to the best of our knowledge) cohort studies [12-14]. The majority of all reported cases of head trauma-related dAVFs are caroticocavernous fistulas, which have a distinct presentation and natural history [15]. There is an added value of traumatic injuries to the presence of fistula alone, which presumably accounts for higher mortality and morbidity, especially in the scenario of intracerebral bleeding; however, it is to be established in future studies.
Trauma-related dAVFs were not directly associated with the severity of recent injury, as presented by Friedman et al. [16], who described a case of progressive, disseminated, posttraumatic dural arteriovenous fistulas resulting in the eventual death of the patient, after minimal head trauma (in contrast to our patient). A 31-year-old man was struck on the head while playing basketball. Two weeks later a soft, pulsatile mass developed on his vertex – a dural arteriovenous fistula. In this case, in the course of five years, multimodal therapy was unsuccessful, the patient suffered from diffuse venous hypertension and fatal multiple intracerebral haemorrhages in both hemispheres.
Overall in our study group five patients had a history of head trauma, complicated by intracranial haemorrhage or tuberculous meningitis, all of which are risk factors for development of dAVFs. Most of these can be attributed to either vascular wall incompetency (posttraumatic or other) or hypercoagulable (even if local, and immune driven) states [4,10-12].
Overall we achieved a 70% angiographic occlusion rate (via arterial feeders), which is comparable to those reported in literature, at 77-95% [17-20]. We used three different embolysates: histoacryl with lipiodol, Onyx-18, and most recently Phil. Although Onyx-18 is regarded as the go-to treatment for cerebral vascular malformations, we had three patients who received a mixed treatment with histoacryl followed by Onyx-18. In two of them > 80% of fistula volume was closed in multiple attempts, while final treatment was ceased due to difficulties in safe catherisation of the feeding arteries. The preference of choosing different agents was based on its availability at the time, with Onyx replacing histoacryl in recent years.
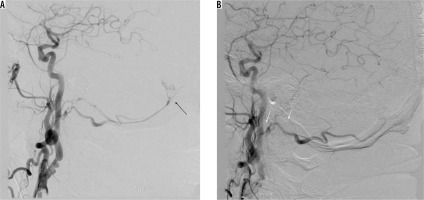
Initial and durable angiographic occlusion rates for embolisation of intracranial dural arteriovenous fistulas are reported to be significantly high for ONYX-18 compared with histoacryl [17-20]. Choo and Shankar [18] found that the chance of not requiring post-embolisation surgery with Onyx-18 was significantly higher than with histoacryl (81.8% vs. 22.2%, respectively). Moreover, patients treated with Onyx-18 tended to have lower complication rates compared with that of histoacryl. There are initial but promising reports regarding Phil, and its safety and efficacy is yet to be evaluated in future studies [21]. We utilised it in one case of a type I fistula and achieved closure on angiography (Figure 1).
Figure 1
Patient with type I fistula (black arrow), presenting as subarachnoid haemorrhage with intraventricular bleeding. Angiographic closure was achieved with PHIL (note the absence of feeding artery – white arrows). On follow-up, there was left abducens nerve palsy
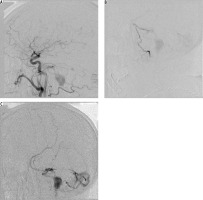
Initial angiographic success may not necessarily lead to long-term cure. Abekar et al. [22] followed 21 patients on angiography after a mean of 14 months post treatment (range 2-39 months). Asymptomatic angiographic recurrence of the fistula was evident in 14.3% of patients. On reviewing the procedural angiograms there was incomplete penetration of the embolic material into the venous portion of the fistula. In a much larger cohort, Gross et al. [2] reported that recurrence or occult residual lesions were seen on subsequent angiography in 3% of cases. In our experience, we had no actual recurrent fistulas; however, we saw partial histoacryl resorption, which may eventually lead to relapse of the fistula. Moreover, one of our patients with type IV fistula, with mild neurological symptoms, initially treated endovascularly, and with most of the fistula volume closed, was lost to follow-up. After two years he was readmitted with subarachnoid haemorrhage attributable to the already progressed fistula (Figure 2).
Figure 2
One of our patients with type IV fistula, with mild neurological symptoms, initially treated endovascularly, with most of the fistula volume closed, was lost to follow-up. After two years he was readmitted with subarachnoid haemorrhage attributable to the already progressed fistula
At our institution, we perform treatment for symptomatic dural arteriovenous fistulas regardless of type, aiming at initial closure if possible, because the natural history of the lesion may depend on the type of presentation and not necessarily its morphology [1,4,7,10,11]. Strom et al. [23] reported that asymptomatic compared to symptomatic fistulas have annual haemorrhage rates of 1.4% versus 19%, respectively. Nevertheless, lower annual haemorrhage rates of asymptomatic dural arteriovenous fistulas (type II and III) [1,4,7,10,11] still pose significant long-term risk to patients. In our experience every fistula poses a significant risk of haemorrhage. In our study type I fistulas accounted for more haemorrhagic presentations that type III and IV combined. There is an additional risk involving the presence of type I fistula because it usually presents itself only due to haemorrhage, remaining unnoticed until that time. It could be the case with posttraumatic fistulas – most of them probably remain undiagnosed when involving draining structures other than the lateral or cavernous sinuses, or unless they bleed.
For asymptomatic patients, we propose justifiable elective treatment if the patient consents to the procedure, after evaluating the anatomy and individual risk of bleeding. Regardless of initial angiographic results or treatment options, long-term angiographic follow-up is highly recommended for all patients [2,22,23].
Spontaneous closure of the dural arteriovenous fistula is rare. Most of the cases involve posttraumatic lesions, which tend to be single, small, type I lesions, often asymptomatic, and remaining unnoticed [24]. Posttraumatic vessel damage could lead to development of scar tissue during the healing process, thus leading to secondary occlusion of the fistula itself. The other mechanism of spontaneous dAVF closure would be intracranial haemorrhage – for non-posttraumatic fistulas. Some authors claimed intracranial haemorrhage could trigger the closure of intracranial arteriovenous malformations, fistulas included, whether linked to haematoma-mediated mass effect or to the secondary vasospasm of the feeding arteries [24,25].
Our study has some limitations. We presented our experience and results of treatment of a relatively small group of patients with intracranial dural arteriovenous fistulas. Our study did not identify a new risk factor of intracranial haemorrhage in intracranial dAVFs, nor did it confirm the previously reported risk factors, probably due to some inhomogeneity in a small population. Nevertheless, we believe our proposition might still be of use if a greater population-based cohort were to be analysed. Moreover, our study showed that we should not underestimate the potential risk of complications of lower type fistulas.
To conclude, regardless of presentation, both symptomatic and asymptomatic dural arteriovenous fistulas deserve clinical attention, structured evaluation, and follow-up. Type I fistulas were associated with haemorrhage in 1/3 of all cases. Overall our results indicate that the risk of haemorrhage and dire consequences is multifactorial.
Conclusions
To conclude, regardless of presentation, both symptomatic and asymptomatic dural arteriovenous fistulas deserve clinical attention, structured evaluation, and follow-up. Type I fistulas were associated with haemorrhage in 1/3 of all cases. Overall our results indicate that the risk of haemorrhage and dire consequences is multifactorial.


