Introduction
Prostate cancer ranks among the most prevalent malignancies affecting men globally, with diverse clinical presentations and outcomes [1]. One of the significant challenges in managing prostate cancer is detecting recurrent disease, especially in patients who have undergone prostatectomy [2]. Early identification of recurrence is paramount for timely intervention and improved patient outcomes. In this context, imaging has been crucial in accurately diagnosing and localising recurrent prostate cancer.
Magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT) have emerged as valuable modalities for detecting recurrent prostate cancer [3]. With its excellent soft tissue contrast and multi-parametric capabilities, MRI offers detailed anatomical information that aids lesion localisation and characterisation. PET/CT, on the other hand, provides functional and molecular insights by utilising radiotracers that target specific cellular and metabolic processes [4].
Among the emerging radiotracers, 18F-fluciclovine has gained prominence for its potential to detect prostate cancer recurrence [5]. 18F-fluciclovine is an amino acid analogue actively transported into proliferating cancer cells, allowing PET imaging of areas with increased cellular activity. This radiotracer’s ability to identify regions of recurrence has led to increased interest in its application, particularly in cases where conventional imaging techniques might fall short [6].
While MRI and 18F-fluciclovine-PET/CT hold promise in recurrent prostate cancer detection [7,8], a critical need exists to evaluate their diagnostic accuracy and comparative performance comprehensively. This study aims to address this gap by systematically assessing the efficacy of these imaging modalities in detecting local recurrence after prostatectomy. By analysing a cohort of patients with suspected recurrence, we aim to determine the strengths and limitations of each modality and shed light on their potential synergistic use in clinical practice.
We discuss the specific objectives of this study, which include evaluating the sensitivity, specificity, positive predictive value, negative predictive value, and overall diagnostic performance of MRI, 18F-fluciclovine PET, maximum standardized uptake value (SUVmax), prostate-specific antigen (PSA), and combinations of these diagnostic modalities. Additionally, we explore the potential of combining these modalities to enhance diagnostic accuracy and offer insights into the optimal approach for recurrent prostate cancer detection. Ultimately, our study aims to contribute valuable insights that can guide clinicians in effectively making informed decisions to manage patients with suspected recurrent prostate cancer.
Material and methods
Patient selection
After obtaining approval from the institutional review board, we accessed the institutional clinical database to identify patients who had undergone both a pelvic MRI and an 18F-fluciclovine-PET/CT scan (Figures 1 and 2). We retrieved 147 patient records and reviewed them to identify cases with clinical or biochemical suspicion of local recurrence. The inclusion criteria specified that patients must have received both imaging studies within 4 weeks. Our study exclusively focused on patients with biochemical recurrence who had undergone prior radical prostatectomy with curative intent. A PSA level of 0.2 ng/ml or greater, followed by another increased measurement at the same level or higher, is defined as biochemical recurrence for patients who underwent radical prostatectomy. From the initial pool, 83 patients were deemed eligible and were included in the analysis. Systemic recurrence was excluded from the study.
Figure 1
A 62-year-old male with post-prostatectomy with false positive fluciclovine uptake in the right prostatectomy bed on axial PET/CT due to urine contamination (A). Axial post-contrast T1-weighted MRI image reveals post-surgical changes (arrow) with no evidence of residual or recurrent disease (B). Subsequent biopsy results were negative for recurrence

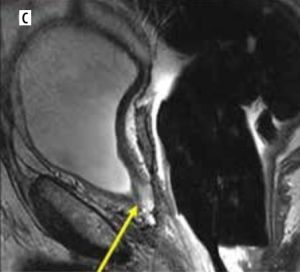
Figure 2
A 73-year-old male with post-prostatectomy with a false negative on axial 18F-fluciclovine-PET/CT (A) study indicated the absence of uptake in the surgical bed. Axial post-contrast T1-weighted (B) and sagittal T2-weighted (C) MRI images exhibit an intermediate T2 signal affecting the resection site of the prostate involving the left posterior anastomosis (arrows) (B and C). Further clinical and imaging follow-up and PSA monitoring confirmed the presence of disease recurrence

Imaging
MRI protocol
All patients were imaged with a 1.5-T or 3.0-T MRI scanner (Optima MR450w, Discovery MR750w, and Discovery MR750; GE Healthcare) using an 8-channel abdominal array coil and an endorectal coil (MR Innerva; MEDRAD). Specifications advanced over the study period but typically included a small field of view; axial, sagittal, and coronal fast spin-echo T2-weighted imaging; diffusion-weighted imaging with b values of 50 s/mm2 and 800 s/mm2 with apparent diffusion coefficient (ADC) reconstruction; and dynamic contrast-enhanced imaging. Whole-pelvis T1-weighted images and diffusion-weighted imaging with ADC reconstruction synthetic diffusion-weighted imaging with a b-value of 1400 s/mm2 were generated using DynaCAD, version 4 (InVivo; Philips Medical Systems). Dynamic contrast-enhanced MRI was performed after the intravenous injection of gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals) at 0.1 mmol/kg of body weight at a rate of 3 ml/s via a power injector; the examination consisted of 29-33 consecutive acquisitions over approximately 3.5 minutes, which allowed complete evaluation of the enhancement characteri-stics of the prostate gland and any lesion. MRI was performed with intravenous contrast, utilising an endorectal coil to enhance imaging quality.
18F-fluciclovine PET/CT
The institution’s 18F-fluciclovine PET/CT protocol complies with the American College of Radiology – American College of Nuclear Medicine guidelines. In brief, except for water, patients fasted for at least 4 hours before injecting approximately 370 MBq (10 mCi) of 18F-fluciclovine. At 5 minutes following the injection, PET/CT imaging was performed from the skull to the mid-thigh to the vertex of the skull. All PET/CT was performed on integrated PET/CT scanners, either on a GE 64-slice Discovery 710 PET/CT scanner or GE Discovery MI 64-slice PETCT (GE Healthcare, Waukesha, Wisconsin, USA) or a Siemens 64-slice Biograph mCT PET/CT scanner (Siemens Medical Systems, Erlangen, Germany) using an institutional standard protocol. Low-dose CT was performed with tube-current modulation with both intravenous and oral contrast. The CT protocol on the GE Discovery 710 or GE Discovery MI scanner was X-ray collimation, 64 × 0.625 mm; pitch factor, 0.984; maximum mA, 560; minimum mA, 60; noise index, 30; gantry rotation time per revolution, 0.5 s; slice thickness, 3.75 mm; and slice increment, 3 mm. The CT protocol on the Siemens 64-slice Biograph mCT was X-ray collimation, 16 × 1.2 mm; pitch factor, 1.4; quality reference mA, 90; dose optimisation index, 3; gantry rotation time per revolution, 0.5 s; slice thickness, 3 mm; and slice increment, 2 mm. The GE and Siemens CT protocols were harmonised over a large cohort of patients to radiation exposure of 3 mGy at a BMI of 25 kg/m2, similar to lung screening CT radiation exposure. Low-dose CT data at the PET resolution in a 70-cm field of view were used for attenuation correction of the PET data in the matrix sizes of 128 × 128 and 200 × 200 for the GE and Siemens scanners, respectively. PET images were acquired at 3 minutes/bed position. The PET data were reconstructed using the following protocols: OSEM 2 iterations, 17 subsets, time-of-flight, point-spread-function correction, 5 mm post-reconstruction Gaussian filtering, matrix size 1256 × 256, reconstruction field of view 70 cm on GE Discovery MI scanner; OSEM 2 iterations, 18 subsets, time-of-flight, point-spread-function correction, 5 mm post-reconstruction Gaussian filtering, matrix size 192 × 192, reconstruction field of view 70 cm on GE Discovery 710 scanners; OSEM 2 iterations, 20 subsets, 6 mm post-reconstruction Gaussian filtering, matrix size 128 × 128, reconstruction field of view 70 cm on the Siemens Biograph mCT scanner.
Recurrence on MRI or positive MRI consists of soft tissue thickening in the surgical bed, enhancing dynamic contrast-enhanced MR with diffusion restriction, enlarged pelvic node, or distant metastasis. At the same time, Fluciclovine avid lesion on PET was considered as positive PET for recurrence.
Reference standard
The results of both MRI and 18F-fluciclovine-PET/CT studies were categorised as positive or negative for recurrence. The SUVmax of intrapelvic lesions was also documented. In total, 27 patients underwent image-guided biopsies for suspicious lesions, and histological findings from these biopsies were considered the reference standard for comparison. For the remaining 56 patients, reference standard determination involved increasing PSA levels, follow-up imaging indicative of recurrence, and physical examinations. All patients diagnosed with recurrence received therapeutic intervention.
Statistical analysis
Patients were characterised concerning the following clini-cal features: Gleason score, PSA at surgery, PSA at follow-up, follow-up MRI result, follow-up PET result, follow-up SUVmax, and disease status (gold standard) based on biopsy for patients who underwent a biopsy at follow-up and a combination of clinical and imaging parameters for patients who did not undergo biopsy at follow-up.
Mean, median, standard deviation, and minimum/maximum values were described for continuous variables, and N (%) for categorical/ordinal variables. Differences in clinical features by evaluation method (biopsy vs. other parameters) were evaluated using t-tests/Wilcoxon rank sum tests for continuous variables and c2/Fisher’s exact tests for categorical variables.
The utility of diagnostic parameters for detecting disease recurrence was assessed using receiver operating characteristics (ROC) analysis to determine the area under the curve (AUC) for each model. Sensitivity, specificity, and positive/negative predictive values were also calculated. Optimal cut-off points for continuous variables were determined based on maximum Youden’s J statistic, defined as (sensitivity + specificity – 1). Analyses were conducted using the overall population and the subset of patients who received biopsies. The covariate combinations were considered: PSA at follow-up, MRI at follow-up, PET at follow-up, SUVmax at follow-up, PET + SUVmax, and PET + SUVmax + PSA.
Equations for predicting the probability of recurrence based on each covariate combination of interest were developed based on the logistic regression models based on the following formula where the inputs included the intercept term (β0) and each covariate coefficient (β1 – βx), where x = number of coefficients.
Results
Descriptive analysis and diagnostic analysis
In the primary analysis involving all 83 patients, the mean post-surgery PSA levels were 11.4 ng/dl (range 0.4 ng/dl to 174.1 ng/dl). The patient characteristics presented for all patients are shown in Table 1. The diagnostic metrics, including sensitivity, specificity, positive predictive value, negative predictive value, and area under the curve (AUC) for MRI, 18F-fluciclovine-PET/CT, SUV, and 18F-fluciclovine-PET/CT + SUVmax are presented in Table 2.
Table 1
Patient characteristics
Table 2
Summary of performance relative to gold standard* (among patients with data on all relevant variables)
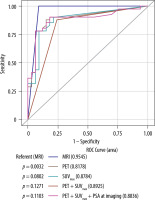
MRI had the highest concordance (96%), sensitivity (100%), specificity (91%), positive predictive value (93%), and negative predictive value (100%) among the diagnostic modalities. MRI exhibited a significantly higher AUC than PET/CT alone (AUC 0.95 vs. 0.81; p = 0.0032). While the AUC was further elevated (0.89), the combined impact of both modalities using the SUVmax cut-off value of 2.85 did not reach statistical significance (p = 0.12) (Figure 3) for detecting recurrence.
Figure 3
ROC curves for diagnosing recurrent prostate cancer in prostatectomy patients. The AUC for MRI was 0.9545, indicating a high discriminatory ability for detecting prostate cancer recurrence. PET and the uptake of the lesion measured by SUVmax, when combined, showed an improved performance compared to using them individually, with an AUC of 0.8925. The combination of PET, SUVmax, and PSA at follow-up had an AUC of 0.8836, slightly lower than the combination of PET and SUVmax alone
Logistic regression models
The model showed that positive finding was the highest predictor of recurrence compared to PSA alone, PET/CT. MR had the largest positive coefficient, suggesting that a positive finding on MRI was a significant predictor for recurrence, β1 = 16.3073 (Table 3).
Table 3
Inputs for predicting recurrence risk for each covariate combination
Discussion
The study’s findings in detecting locally recurrent prostate cancer suggest limited added value of PET/CT compared to MRI, given the latter’s higher specificity. However, implementing a higher SUVmax cut-off value (> 2.85) demonstrated enhanced specificity and accuracy for PET/CT. Moreover, combining MRI and 18F-fluciclovine-PET/CT presented an added benefit, potentially offering a comprehensive strategy for assessing disease recurrence. The substantial improvement in AUC values underscores the diagnostic utility of this combined approach. These results emphasise the importance of leveraging the strengths of both modalities in the clinical evaluation of locally recurrent prostate cancer.
Imaging is pivotal in diagnosing and managing prostate cancer, a common malignancy affecting men worldwide [9]. Accurately detecting recurrent prostate cancer, particularly in the post-prostatectomy setting, remains challenging due to the potential overlap between physio-logical postoperative changes and disease recurrence. This study aimed to assess the diagnostic accuracy of 18F-fluci-clovine-PET/CT compared to magnetic resonance imaging (MRI) for detecting recurrent prostate cancer after prostatectomy. The findings from this study could contribute significantly to the ongoing discourse surrounding the optimal imaging approach for this complex clinical scenario of recurrence.
The primary analysis of this study demonstrated that MRI exhibited a markedly higher diagnostic accuracy than PET/CT. The area under the curve (AUC) values for MRI and PET/CT were 0.95 and 0.82, respectively. This observation aligns with the established advantages of MRI in offering excellent soft-tissue contrast and high spatial resolution [3,10]. The detailed anatomical information MRI provides facilitates the precise identification of suspicious lesions and differentiation between postoperative changes and disease recurrence. MRI’s superior tissue characterisation capabilities, especially in evaluating soft tissues, make it an indispensable imaging modality for assessing prostate cancer recurrence.
In contrast, while PET/CT has shown potential in detecting prostate cancer recurrence through visualisation of metabolic activity, the study’s results suggest that its diagnostic accuracy might be suboptimal. The AUC of 0.8178 for PET/CT indicates that the modality alone might be less reliable in distinguishing between postoperative changes and recurrent disease. These findings concur with previous research highlighting the challenges of using PET/CT to detect local prostate cancer recurrence [5,11]. False positives can occur due to acute and chronic inflammation (including post-radiation inflammation) and infection [6]. Recurrent disease at the anastomosis may be close to the bladder and obscured due to the intense activity from the bladder. The study’s relatively low specificity of 76% underscores the need to refine the criteria for interpreting PET/CT images.
Interestingly, the study explored the potential of a combined imaging approach by introducing a cut-off value for SUVmax, a measure of metabolic activity. The results revealed that this hybrid approach improved specificity and overall diagnostic accuracy. The AUC of 0.89 for PET/CT + SUVmax (cut-off of 2.85) indicates that this combined approach yielded better diagnostic performance than PET/CT alone. This finding aligns with recent research highlighting the potential benefits of multimodal imaging strategies to enhance diagnostic accuracy [12]. Using SUVmax as a threshold to enhance specificity is essential because it addresses one of the challenges associated with PET/CT: false positives [13].
The subset analysis involving patients who underwent biopsy provided further insights into the performance of both imaging modalities. MRI and PET/CT demonstrated high sensitivity and negative predictive value, indicating their ability to identify patients with recurrent disease accurately. However, the study identified a limitation in terms of specificity, with MRI exhibiting 91% specificity and PET/CT only 76%. This suggests that while these modalities excel in correctly identifying positive cases, they are also prone to false positives. False positive results can lead to unnecessary invasive interventions. The introduction of SUVmax as a criterion significantly improved the specificity of PET/CT to 85%, addressing a key limitation.
Recent literature further supports the discussion surrounding the diagnostic accuracy of 18F-fluciclovine-PET/CT and the potential benefits of combining imaging modalities. A similar evaluation of 18F-fluciclovine-PET/CT’s diagnostic accuracy in detecting local recurrence and its findings echoed the current study’s results in terms of sensitivity and specificity [5]. The study emphasised the importance of integrating modalities for optimal outcomes, aligning with the current study’s observations. Similarly, SUVmax enhances the specificity of 18F-fluciclovine-PET/CT [13,14]. This research supports the current study’s observation on the potential advantages of using SUVmax as a criterion to improve the diagnostic accuracy of PET/CT.
This study offers valuable insights into the diagnostic performance of 18F-fluciclovine-PET/CT and MRI in detecting locally recurrent prostate cancer after prostatectomy. The findings underscore the strengths of MRI in providing detailed anatomical information and higher AUC values. The study also demonstrates the potential of PET/CT, especially when coupled with SUVmax criteria, to improve specificity and overall diagnostic accuracy. The combined approach of integrating both imaging modalities holds promise for achieving heightened diagnostic accuracy, particularly in distinguishing recurrent disease from benign conditions. Recent relevant literature further bolsters the study’s findings, emphasising the significance of combining imaging modalities to enhance diagnostic capabilities in cases of recurrent prostate cancer.
Similar to 18F-Fluciclovine radiotracer, 68Ga-prostate specific membrane antigen (PSMA)-11, and 18F-piflufolast are the PSMA-based radiotracers used in PET/CT and are approved by the United States Food and Drug Administration [15]. A few studies, including patients with biochemical recurrence after radical prostatectomy, noted that 68Ga-PSMA PET/CT has a superior detection rate to 18F-fluciclovine PET/CT, especially with PSA levels less than 1.0 ng/ml [16,17]. However, compared to MRI, PSMA PET/CT has lower sensitivity (64% vs. 91%) in detecting cancer recurrence, particularly in the prostatic fossa [18]. This is due to the PSMA excretion through urine, which may obscure the bladder lumen, bladder neck, and vesicourethral junction, thereby limiting the visualisation of recurrent prostate cancer [15].
The heterogeneity in PSA concentrations among patients with local recurrence of prostate cancer was observed in the study. The study’s primary analysis involved 83 patients, with the mean post-surgery PSA levels being 11.4 ng/dl, but the range was quite broad, spanning from 0.4 ng/dl to 174.1 ng/dl. This heterogeneity in PSA levels among patients with prostate cancer can be attributed to several factors: biological variability, treatment responses, disease progression, technical and methodological variations, and individual patient factors [19-24].
Limitations
The study’s cohort consists of a relatively small sample size, which could limit the generalisability of the findings. A larger sample size might provide a more robust representation of the population and enhance the statistical power of the analysis. The study was conducted at a single institution, which could introduce potential selection bias and limit the diversity of patient populations and disease presentations. Multi-centre studies involving various clinical settings and patient demographics would provide a more comprehensive perspective. The exclusion of patients with indeterminate Fluciclovine uptake might introduce bias because these cases could influence the overall diagnostic accuracy and performance of 18F-fluciclovine-PET/CT. The study uses different reference standards for comparison – pathology for the subset of patients who underwent biopsy and clinical criteria for the remaining patients. This difference in standards might introduce variability in the assessment of diagnostic accuracy and complicate direct comparisons between the 2 modalities.
The study’s reliance on a relatively short follow-up period (within 4 weeks of imaging) might not capture the full spectrum of disease recurrence. Long-term follow-up data could provide a more accurate assessment of the diagnostic performance of the modalities over time. While the study focuses on diagnostic accuracy, it needs to address the cost-effectiveness of the imaging modalities. The clinical utility of these techniques depends on their accuracy, cost implications, and potential impact on patient outcomes.
Both MRI and 18F-fluciclovine-PET/CT can be influenced by operator expertise and variability in interpretation. The study does not explicitly address potential sources of interobserver and intraobserver variability in the imaging results.
Conclusions
In evaluating locally recurrent prostate cancer post-prostatectomy, this study demonstrates that 18F-fluciclovine-PET/CT provides limited additional diagnostic benefit compared to MRI. While both modalities show potential, MRI stands out with a higher specificity. The specificity and accuracy of 18F-fluciclovine-PET/CT can be improved when applying a cut-off value of SUV > 2.85. Furthermore, combining both imaging modalities and including the PSA values offers an added advantage in assessing recurrence, suggesting a potential complementary role in clinical practice. These findings underscore the ongoing need for more extensive, multi-centre studies with extended follow-up periods to elucidate further the effectiveness of these modalities and their use in detecting recurrent prostate cancer.