Introduction
Hemifacial spasm (HFS) is a neuromuscular movement disorder characterised by involuntary, paroxysmal twitching of facial muscles on one side of the face, innervated by the ipsilateral facial nerve [1,2]. A significant community-based study conducted in the US estimated that the prevalence of HFS was 7.4 per 100,000 in men and 14.5 per 100,000 in women [3]. The psychosocial stress associated with this non-life-threatening condition necessitates timely diagnosis and treatment. Most cases are due to irritation of the facial nerve by an ectatic or aberrant vascular structure at the root exit zone [4]. Space-occupying lesions of the cerebellopontine angle constitute a rare cause of HFS [1]. HFS due to vascular compression can be treated with medications like carbamazepine, botulinum toxin, and microvascular decompression.
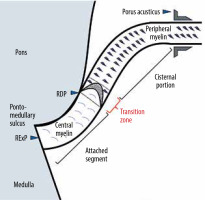
The transition from central myelin produced by glial cells to peripheral myelin synthesised by Schwann cells constitutes the nerve’s root entry/exit zone (REZ), the most vulnerable site for compression of all cranial nerves [5,6]. The facial nerve emerges from the brainstem at the root exit point (RExP) within the pontomedullary sulcus. As the attached segment (AS), it traverses along the undersurface of the pons for 8 to 10 mm. The facial nerve then separates from the undersurface of the pons at the root detachment point (RDP). The segment of the facial nerve that extends 2 to 4 mm from the RDP is the transition zone (TZ) or Obersteiner-Redlich zone. The REZ segment extends from the RDP to 4 mm distal to RDP and includes TZ. The cisternal portion (CP) extends anterolaterally up to the porus acusticus [7,8]. Vascular contact from the RExP to the end of the TZ is associated with the HFS, and persistent symptoms after surgery have been linked to insufficient decompression of this proximal vulnerable segment [7,9].
However, fewer data establish the prevalence of vascular contact of facial nerves in patients without HFS. Our study aims to assess the prevalence of vascular contact of facial nerves in patients without HFS on MRI.
Material and methods
A retrospective study was conducted, and the need for informed consent was waived. Consecutive patients who underwent an MRI brain scan for various indications were assessed from our radiology database. Adult patients (age ≥ 18 years) who underwent high-resolution constructive interference in steady-state (CISS) sequence of the brainstem and posterior fossa with predominant imaging indications of headache, audiovestibular, and ocular symptoms were included in the study. Patients with a history of hemifacial spasm/facial palsy, traumatic brain injury, intracranial tumour, intracranial surgery, trigeminal neuro-vascular compression, brain radiation therapy, and studies with poor image quality and without CISS sequence were excluded. The MR imaging of 112 patients (224 sides) who fulfilled the eligibility criteria was reviewed by 2 independent radiologists.
Studies were performed using the standard MRI brain protocol, which included T1WI and T2WI in all 3 planes, axial FLAIR (fluid-attenuation inversion recovery), DWI (diffusion-weighted imaging), SWI (susceptibility-weighted imaging), and other specific sequences according to history such as coronal FLAIR for the history of seizures and high-resolution thin-section volumetric CISS (constructive interference in steady state) sequence were acquired with one of the 2 MRI scanner platforms as follows:
1.5 Tesla Siemens Magnetom Avanto MR system (Siemens AG, Berlin/Munich, Germany) – axial CISS sequence (constructive interference in steady state; images through the internal auditory canals and brainstem: TR = 1200 ms, TE = 268 ms; flip angle = 150 degrees, matrix = 320 × 256, FOV = 240 mm, no saturation, 0.7 mm axial slice thickness with coronal and sagittal reformatted images).
1.5 Tesla United Imaging uMR-570LH MR system (United Imaging, Houston, Texas, North America) T2 axial SPAIR_CISS sequence (spectral attenuated inversion recovery constructive interference in steady state images through the internal auditory canals and brainstem: TR = 1300 ms, TE = 240 ms; flip angle = 150 degrees, matrix = 352 × 317, FOV = 180 × 200 mm, no saturation, 0.68 mm axial slice thickness with coronal and sagittal reformatted images).
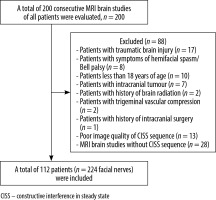
A total of 200 consecutive MRI brain studies were evaluated. Patients with a history of hemifacial spasm/facial palsy (n = 8), traumatic brain injury (n = 17), intracranial tumour (n = 7), brain radiation treatment (n = 2), trigeminal vascular compression (n = 2), intracranial surgery (n = 1), paediatric cases (n = 10), poor image quality (n = 13), and absent CISS sequence (n = 28) were excluded, and a total of 112 patients were evaluated (Figure 1).
The reviewing radiologists were aware of the study objectives but were blinded to the clinical history of hemifacial spasm. They reviewed all the MRI pulse sequences and multiplanar reconstructions. They assessed the facial nerve for the presence of vascular contact, the number of contact points, the location of contact along the intracranial course of the facial nerve, the culprit vessel, and the severity of compression. Because comparison with the intra-operative gold standard was not possible in these patients, only the neurovascular contacts with a high level of certainty were considered and further characterised. The location of vascular contact along the intracranial course of the facial nerve identified was classified as “brain stem”, “REZ”, or “CP” based on anatomic landmarks that are consistently visualised with MRI, as proposed initially by Tomii et al. in 2003 [8] and later expanded by Campos-Benitez and Kaufmann [7] (Figures 2 and 3). The brain stem segment of the facial nerve extends from the RExP at the pontomedullary sulcus to the RDP and includes the attached segment (AS) of the facial nerve along the undersurface of the pons for 8 to 10 mm. The REZ segment extends from the RDP to 4 mm distal to the RDP and includes TZ wherein central glial myelin changes to peripheral myelin produced by Schwann cells. CP extends distal to the RDP up to the porus acusticus. Neurovascular contact was considered in the absence of cerebrospinal fluid (CSF) cleft between the vessel and the nerve. The number of points of neurovascular contact was also recorded. The scale proposed by Campos-Benitez and Kaufmann was used to grade the vascular contact, which defined mild as “contact without indentation of the facial nerve”, moderate as “indentation of the facial nerve without deviation of its course”, and severe as “deviation of the natural course of the facial nerve” [7].
Figure 2
Normal anatomy of the intracranial course of the facial nerve. A) Oblique coronal high-resolution T2-weighted constructive interference in steady state (CISS) sequence demonstrates the intracranial anatomy of the facial nerve on a single image. Root exit point (RExP; white arrowhead), root detachment point (RDP; white arrow), estimated lateral border of transitional zone (TZ; red arrow) and cisternal portion (CP). B) Axial high-resolution T2-weighted CISS sequence illustrating root entry/exit zone (dashed white line) which includes RDP and TZ. C) Coronal high-resolution T2-weighted CISS sequence illustrating Brainstem segment (dashed white line), which includes RExP and attached segment
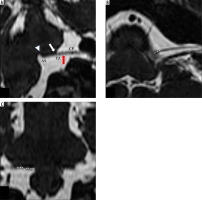
Figure 3
Normal anatomy of the intracranial course of the facial nerve.
RExP – root exit point, RDP – root detachment point
Patient demographics were described using descriptive statistics. The Kolmogorov-Smirnov test was used to check the normal distribution of the data. Standard deviation (SD) and mean were used to express continuous variables. Ordinal or categorical variables are expressed in frequency (n) and percentage (%). The k coefficient of agreement index, which determines the degree of agreement compared with chance, was used to compute interobserver agreement for the presence or absence of nerve contact. The k result was interpreted as follows: values ≤ 0 indicate no agreement, 0.01-0.20 none to slight, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 substantial, and 0.81-1.00 as almost perfect agreement [10]. The p-values < 0.05 were considered statistically significant.
Results
The cohort had a mean age of 42 years (range: 18-81 years), with 42 men (37.5%) and 70 women (62.5%). The most common indication for MRI brain was headache (34.8%), followed by ocular (8.0%) and audio-vestibular symptoms (7.1%), and seizures (6.3%). The results of the assessment of neurovascular contact of the facial nerve are reported in Table 1.
Table 1
Results of the assessment of neurovascular contact of the facial nerve
The first radiologist identified vascular contact in 114 of 224 facial nerves (50.9%), with the CP being the most common location of contact (56.6%) (Figure 4), followed by the REZ (38%) (Figure 5), and lastly the brainstem (5.4%) (Figure 6). The most common culprit vessel was the anterior inferior cerebellar artery (AICA), reported in 67.4% of cases. The severity of vascular contact in most cases was mild (78.3%).
Figure 4
Moderate neurovascular contact between the left anterior inferior cerebellar artery and the cisternal portion of the facial nerve. Axial (A) and coronal oblique (B) high-resolution T2-weighted constructive interference in steady state sequences demonstrate indentation of the cisternal portion of the left facial nerve (white arrow) by the anterior inferior cerebellar artery (red arrow) suggestive of moderate neurovascular contact
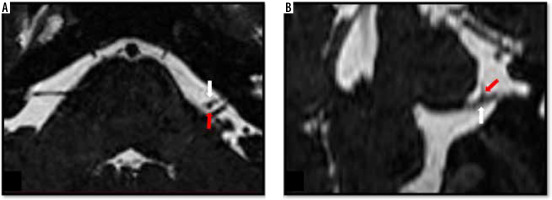
Figure 5
Moderate neurovascular contact between the right posterior inferior cerebellar artery and the facial nerve. Coronal (A) and coronal oblique (B) high-resolution T2-weighted constructive interference in steady state sequences demonstrate indentation (moderate neurovascular contact) of the right facial nerve at the level of root entry/exit zone (white arrow) by the posterior inferior cerebellar artery (red arrowhead)
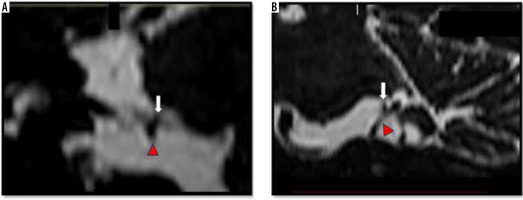
Figure 6
Neurovascular contact involving the brainstem segment of the left facial nerve. Coronal oblique (A) and sagittal (B) high-resolution T2-weighted constructive interference in steady state sequences demonstrate the left anterior inferior cerebellar artery (white arrow) indenting (moderate neurovascular contact) the attached segment (Brainstem segment) of the left facial nerve along the undersurface of the pons
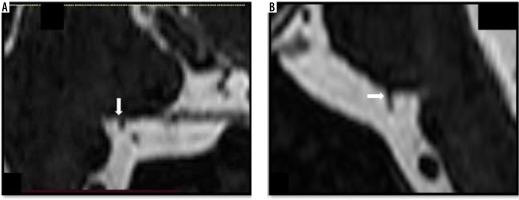
The second radiologist identified vascular contact in 107 of 224 facial nerves (47.8%), with the CP being the most commonly affected segment (58.2%). Vascular contact was most commonly caused by AICA, which occurred in 67.2% of cases. The severity of vascular contact was mild in 76.2% of cases. Severe neurovascular compression was not identified in any of the cases by either of the radiologists. The neurovascular contact involving the facial nerve at 2 points (Figure 7) was identified in 13.2% of cases by the first radiologist and in 14.0% of cases by the second radiologist.
Figure 7
Multiple neuro-vascular contacts between the loop of the anterior inferior cerebellar artery and the right facial nerve. Axial (A, B) and coronal (C) high-resolution T2-weighted constructive interference in steady state sequences demonstrate contact of the right facial nerve at the level of root entry/exit zone (white arrow) in Figure A and indentation of the cisternal portion (yellow arrow) by the anterior inferior cerebellar artery loop (red arrow) shown in Figures B and C.
In 108 of 224 facial nerves (48.2%), both radiologists agreed that there was no vascular contact. Therefore, the prevalence of vascular contact in asymptomatic patients may range from 47.8% to 50.9%. Table 2 shows the interobserver agreement between the 2 radiologists. The k coefficient of agreement for the presence or absence of vascular contact was 0.90 (95% confidence interval = 0.84-0.95; standard error = 0.028), indicating almost perfect agreement between the 2 radiologists.
Discussion
The prevalence of HFS is 9.8 per 100,000 people, and the average age of onset is 44 years [11]. Among various causes of HFS, vascular contact is considered to be the most common aetiology, which can be effectively cured by microvascular decompression, with success rates exceeding 90% for the initial surgery [5,12,13]. Facial nerve-vascular contact can be seen even in asymptomatic patients, the prevalence of which is not well documented in the literature. Deep et al. [6] encountered a prevalence as high as 53% in asymptomatic patients, with the anterior inferior cerebellar artery (AICA) as the most common culprit vessel, with predominant peripheral (cisternal portion) and mild severity of contact. However, they did not consider multiple points of contact.
A few studies conducted in the cohort of HFS encountered vascular contact on the asymptomatic contralateral side of these patients. Vascular contact of about 15% was seen on the contralateral asymptomatic side of patients with HFS [14-16]. In a study published by Tash et al. [17] in 1991, vascular contact was encountered in all 13 patients with HFS and in 21% (70 patients) of asymptomatic patients. Because this study was conducted in the early generation of MRI with 3 mm thick coronal T1-weighted images in the control group, there is a strong possibility of underestimation of the prevalence of neurovascular contact. Kakizawa et al. [18] demonstrated a high prevalence of contact (78.6%) of the facial nerve with other structures like arteries, veins, dura mater, and other cranial nerves, and recorded the distance from the TZ instead of anatomic segments. The high prevalence noted in this study might be attributed to the inclusion of neurovascular contact in the meatal segment of the facial nerve. Sekula et al. [19] described neurovascular compression of the proximal vulnerable segment in 4 out of 28 asymptomatic control groups. Ozan et al. [20] conducted a study to evaluate the contact patterns of the facial nerve in 52 patients with Bell’s palsy and 25 healthy controls. They concluded that multiple vascular contacts were seen on symptomatic sides. In 2020, Traylor et al. [21] conducted a study on 330 patients with HFS and reported neurovascular contact in 97.88% of symptomatic sides and 38.79% of asymptomatic sides. Symptomatic sides showed more severe grades of compression caused by an artery in the proximal susceptible portion.
Because we are limited by the lack of gold standard surgical confirmation of neurovascular contact, which is not possible in these asymptomatic patients for obvious reasons, we have compared our results with the study [7] involving intra-operative observations in HFS patients, and the following conclusions can be drawn:
Mild compression was more prevalent in our study, which is in agreement with Deep et al. [6], whereas moderate or severe compression was commonly seen in patients with symptoms of HFS [7]. Thus, logically explaining the contact without indentation of the nerve, which is termed “mild”, would be less likely to cause symptoms.
In asymptomatic patients, neurovascular compression is frequently seen in the distal segments, such as CP, which agrees with Deep et al. [6]. This contrasts with the studies on patients with HFS, wherein compression was demonstrated in proximal segments from the RExP at the pontomedullary sulcus to the end of TZ. The distal part of the nerve with peripheral Schwann cell synthesised myelin is more resistant to pulsatile vascular compression, the reason for which is not well understood. The central nervous system segment of the cranial nerves is hypothesised to be more susceptible to microtrauma and structurally weaker than the periphery part [22]. Histological studies on cranial nerves (trigeminal, facial, glossopharyngeal, and vagus nerves) demonstrated that the longer the central myelin portion of the nerve, the greater the incidence of symptoms (trigeminal neuralgia, HFS, vagal, and glossopharyngeal neuralgias) and thus support this hypothesis [23,24]. These studies infer that the neurovascular conflict encountered in the proximal segments of the facial nerve is more likely to be symptomatic. In our study, 42.6% (37.5% in the root entry/exit zone and 5.1% in the brainstem segment) of neurovascular contacts were seen in the proximal segment of the facial nerve. Deep et al. [6] also showed significant proximal neurovascular contacts of about 48.3% (35.3% in the root entry/exit zone and 13.0 % in the brainstem segment). Contact involving the brainstem segment was 5.1% in our study.
AICA was the most common culprit vessel, seen in 67.3% of our study and 55.6% in the study by Deep et al. [6], followed by VOUO (28.3%), PICA (2.8%), and then VA (1.6%) (Figure 8). The anatomy of AICA, which makes a “hairpin” loop within the cerebellopontine angle cistern and internal acoustic canal, results in a higher probability of contact with the resistant cisternal portion [6].
In addition, we encountered 2 points of neurovascular contact in 13.6% of asymptomatic sides. Multiple culprit vessels were seen in 38% of cases who underwent microvascular dissection surgery [7]. Ozan et al. [20] found multiple vascular contacts on the affected sides in facial palsy and assigned type 3 (presence of multiple vascular contacts) vascular contact pattern of the facial nerve to 34 (65.4%) symptomatic sides of patients with Bell’s palsy, 10 (19.2%) contralateral asymptomatic sides, and 5 (10%) to the symptomatic control group. Multiple vascular contacts were a main predisposing risk factor in the development of Bell’s palsy [20].
Knowledge regarding the vulnerable segment of the facial nerve, the number of points of contact, the severity of contact, and the prevalence of neurovascular contact in asymptomatic patients is pivotal in preoperative planning. This might be particularly helpful in patients with prior failed microvascular decompression. The central myelinated vulnerable proximal segment should always be given a careful final look, especially in patients presenting with hemifacial spasm, because there can be multiple points of contact due to the high prevalence of asymptomatic distal neurovascular contact. According to current research, decompression of the entire proximal segment results in the best surgical outcome [6,7,9,22,25]. The major limitation of our study is the lack of surgical correlation and confirmation of neurovascular contact, which is the gold standard. Surgical confirmation in asymptomatic patients was also not possible.
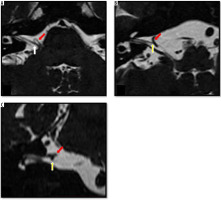
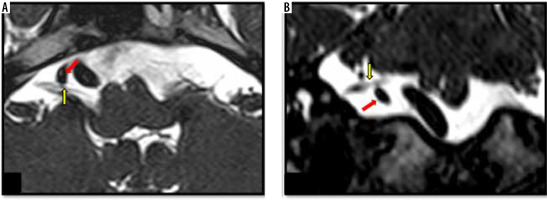
Figure 8
Mild neurovascular contact between an ectatic right vertebral artery and the cisternal portion of the facial nerve. Axial (A) and coronal (B) high-resolution T2-weighted constructive interference in steady state sequences demonstrate contact (mild neurovascular contact) between the cisternal portion of the right facial nerve (yellow arrow) and the ectatic right vertebral artery (red arrow)
Conclusions
The prevalence of neurovascular contact of the facial nerve in patients asymptomatic for hemifacial spasms is as high as 51%. Typically, one point of contact was encountered, with AICA being the most common culprit vessel. The cisternal portion of the facial nerve was frequently affected, with mild to moderate severity. This contrasts with the studies conducted on patients with HFS, wherein the pulsatile neurovascular conflict occurred more proximally (brainstem and root entry/exit zone including transitional zone). The results in asymptomatic patients should be considered in evaluating the candidacy of HFS patients for microvascular decompression.