Introduction
Breast cancer is by far the most frequent cancer in women and the most frequent cause of cancer death in developing countries. Mammography is the standard investigation for screening of breast cancer, and it is performed as a diagnostic investigation in cases of a palpable lump. However small masses may be obscured in dense fibro-glandular parenchyma, a problem that is more pronounced in dense breasts. Therefore, mammographic density (MD) is an integral part of the mammographic report. The MD evaluation can be done with subjective visual assessment, semi-automated methods, and automated methods [1]. Visual assessment is guided by the Breast Imaging – Reporting and Data System (BIRADS) given by the American College of Radiology (ACR), where the breast is categorised into 4 compositions based on the relative fatty and fibro-glandular tissue, with category A being entirely fatty and category D being the most dense. MD can also be assessed on automated area-based options, or volumetric assessment can be done. Better reproducibility is found with newer methods based on automated volumetric density measurement [1], such as Volpara® (Volpara Solutions) and Quantra® (Hologic, Danbury, Conn).
Besides the fact that masses may be obscured by mammographic density, it is also a well-known fact that high mammographic density is a risk factor for developing breast cancer. Based on the expression of oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2), breast cancer is further classified into subtypes [2] such as ER positive, PR positive, HER2 positive, and triple-negative breast cancer (TNBC). The expression of these receptors has therapeutic and prognostic implications.
Although numerous studies have shown that women who have high breast density on mammogram have increased risk of breast cancer when compared with women with lower breast density, the relationship between mammographic breast density and hormone receptor status is less clearly defined, with variable results reported by various authors.
In this study, we aimed to investigate potential association between mammographic density measurements with hormone receptor status of breast cancer in Indian women.
Material and methods
The study was performed after obtaining approval from the Institutional Ethics Committee (IEC), and informed consent was taken from all participants. The study was conducted over a period of 12 months. All patients with suspected breast cancer referred for mammography from the Department of Surgery, Lok Nayak Hospital, were evaluated, and those who were confirmed to have breast cancer on histopathology were included in the study. Exclusion criteria were patients with a diagnosis other than breast cancer on histopathology, skin disorder/inflammatory conditions of breast or ulcerative lesions in the breast precluding a mammogram, pregnant or lactating patients, and patients who had undergone any breast surgery on the side to be evaluated. Patients were also excluded from the study if they had received chemotherapy or radiotherapy. Thus, a total of 100 histopathologically proven breast cancer cases were included in the study.
Clinical details of patients including age, history of breast lump, pain, nipple discharge, nipple and skin retraction were noted. Relevant history including family history of breast cancer, age at menarche, age at first childbirth, number of children, and menopausal status were also noted.
Mammography was performed on a Hologic Selenia dimensions system. Two views of each breast were obtained: medio-lateral oblique (MLO) and cranio-caudal view (CC). The density of breast contralateral to the mass (to avoid compounding effect of the mass) was assessed using Hologic Quantra volumetric computerised software version 2.1.1, which determined the area breast density (ABD) as the ratio of the area of fibro-glandular tissue (Figure 1). The software estimates the percentage of volumetric breast density based on X-ray attenuation from fat and dense tissue on the mammograms. It then provides the corresponding area breast density calculated from the volumetric density measurement [3].
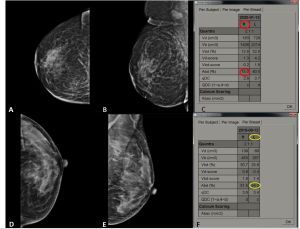
Figure 1
Depiction of area breast density (ABD) using Hologic Quantra volumetric computerised software: cranio-caudal (CC) and medio-lateral oblique (MLO) views of right breast (A and B) in a patient with left breast mass shows ABD of 15% (low breast density) (C). CC and MLO views of left breast (D and E) in another patient with right breast mass showing measured ABD value of 54.3%, i.e. high breast density (F)
Morphological features of the masses were also studied. Ten patients had ulcerative growth on the affected breast; in these patients the mammogram was acquired only on the contralateral side. Hence, the morphological features of 90 lesions were evaluated on mammograms, while mammographic breast density (ABD) evaluation was done in 100 patients on the contralateral side. Patients having ABD less than or equal to 24.9% were categorised as having low ABD, and those having ABD more than 25% were categorised as high ABD (see Figure 1), as suggested by Jeffers et al. [4].
Histopathological diagnosis was established as infiltrating ductal carcinoma (IDC) in all the patients. Further immunohistochemistry was done for expression of hormone receptor status, i.e. ER, PR, and HER2neu, and the data were recorded.
Statistical analysis
Statistical Package for the Social sciences (SPSS) version 25.0 was used for statistical analysis. The quantitative variables were expressed as mean ± SD, while the qualitative variables were described as frequencies/percentages. Continuous variables such as mean age, among low- and high-density breasts, were analysed with unpaired t-test. The χ2 test was used for nominal data such as assessing calcifications among various hormone receptor status. The chi-square test was also used for comparison of low and high breast density with hormone receptor status, while mean ABD and hormone receptor status were evaluated with unpaired t-test. A p-value < 0.05 was considered statistically significant.
Results
The study included 100 patients with histopathologically confirmed infiltrating ductal carcinoma (IDC) between 25 and 68 years of age. Most of the patients had at least one child (95% of cases). 67% patients achieved menarche at or before 13 years of age. Most of the patients were post-menopausal (67%). On analysis of hormone receptor status, multiple receptors were positive in several patients: 41% of patients had ER-positive tumours, 33% of patients had PR-positive tumours, and 34% of patients had HER2-positive tumours.
Using the ABD provided by Hologic Quantra computerised software version 2.1.1, 58% of patients had low breast density (ABD less than 25%), while 42% of patients had high breast density (ABD more than 25%). The mean age of patients with low density breast was 51 years, and that for patients with high density breast was 47 years; however, the difference was not statistically significant. Similarly, we did not find any difference in parity between the 2 groups.
Of the100 patients included in the study, the morphological features of mass lesions were evaluated in 90 patients. Irregular shape with indistinct margin was the most common, seen in 43% of cases, followed by spiculated margins (39%). We observed that irregular shape with spiculated margins was most common in HER2-positive tumours, being seen in 51.5% of patients, while it was seen in 38% and 32% of ER-positive and PR-positive tumours, respectively. However, this difference failed to reach statistical significance.
In our study, out of the 90 patients with masses, 57 (i.e. 63%) patients had calcifications, of which fine pleomorphic in grouped distribution was most commonly seen (36% of all cases). Calcification was more commonly seen in HER2-positive tumours (28/33;85%), compared to HER2-negative tumours (29/57; 50%), and the difference was found to be statistically significant (see Figure 2 and Table 1). However, for ER status, there was no significant difference in the presence of calcification between ER-positive and -negative tumours (58% vs. 67%). This was true for PR status as well with calcification, seen in 64% of cases with PR-positive tumours and 62% cases with PR-negative tumours.
Table 1
Comparison of calcifications with hormone receptor status of breast cancer
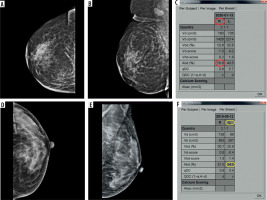
Figure 2
Calcifications in HER2-positive tumour: medio-lateral oblique (MLO) views of bilateral breasts (A and B) showing an irregular high-density mass with indistinct margins in upper half of left breast, with associated adjacent architectural distortion, showing fine pleomorphic calcifications within (better depicted in inset)
On comparison of the hormone receptor status between the patients with high and low ABD, we observed that of the 42 patients having high breast density (ABD > 25%), ER-positive status was seen in 23 patients (55% [23/42]) (Figure 3), while 28.5% (12/42) of patients had PR-positive tumours and 35% (15/42) of patients had HER2-positive tumours. Out of 58 patients with low-density breast (ABD < 25%), 18 (31% [18/58]) had ER-positive tumours, 36% had PR-positive tumours (Figure 4), and 33% of patients had HER2-positive tumours (Figure 5). Thus, patients with high breast density more often had ER-positive tumours compared to those with low ABD, and the difference was statistically significant (Table 2). However, the same was not observed for PR and HER2 receptor status.
Table 2
Comparison of area breast density (ABD) with hormone receptor status of breast cancer
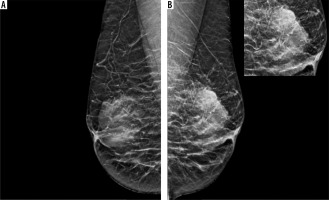
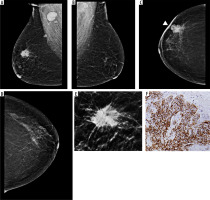
Figure 3
ER-positive tumour in a 59-year-old patient with high density breast: medio-lateral oblique (MLO) and cranio-caudal view (CC) views (A to D) showing an irregular high-density mass with obscured margins in upper inner quadrant, extending to retroareolar region of right breast (marked with arrows in A and C). Associated skin thickening (arrowheads) and axillary lymphadenopathy also seen. Histopathology displaying infiltrating duct carcinoma (IDC) on haematoxylin and eosin staining with strong ER expression on immunohistochemistry (E and F, respectively); PR and HER2 receptor expression was not seen. Area breast density (ABD) of left breast was 47.7%. The patient underwent neoadjuvant chemotherapy followed by modified radical mastectomy and received adjuvant chemo/radiotherapy and hormone therapy
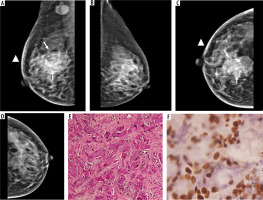
Figure 4
PR-positive tumour in a 63-year-old patient with low-density breast: medio-lateral oblique (MLO) and cranio-caudal view (CC) views (A to D) showing an irregular high-density mass in upper outer quadrant of left breast with spiculated margins. Associated architectural distortion and peri-areolar thickening seen (arrowheads). No pathological calcification seen within the mass. Immunohistochemistry showing strong expression of PR receptor (E). Area breast density (ABD) of right breast was 10%. The patient underwent breast conservation surgery followed by chemoradiotherapy and hormone therapy
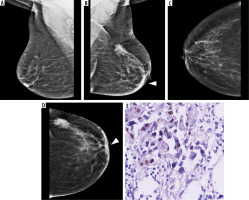
Figure 5
HER2-positive tumour in a 56-year-old patient with low-density breast: medio-lateral oblique (MLO) and cranio-caudal view (CC) views (A to D) showing an irregular high-density mass in upper outer quadrant of right breast with spiculated margins (magnified view in E). Associated focal skin thickening (arrowhead) and axillary lymphadenopathy seen. Immunohistochemistry showing diffuse strong positivity for HER2 neu (F). Area breast density (ABD) of left breast was 16.2%. The patient underwent breast conservation surgery followed by chemoradiotherapy
Upon analysing the ABD status among ER-positive receptor status, we observed that the mean ABD in patients with ER-positive status was 27% ± 14% while the ABD in patients with ER-negative tumours was 23.24% ± 12.11%, and the difference is statistically significant, p-value 0.01. The mean ABD in various receptor statuses is depicted in Table 3. For PR and HER2 receptor, no significant difference in mean ABD of positive and negative receptor status was seen.
Table 3
Comparison of mean area breast density (ABD) with hormone receptor status of breast cancer
Discussion
Mammography is an established technique for investigation of breast disease [5]. Mammograms represent a planar two-dimensional (2D) view of the three-dimensional (3D) structure of the breast [6]. Along with the detection of masses, it also displays their morphological features, including the margins and calcifications, with high accuracy. Mammographic density (MD) reflects the composition of fibro-glandular and fat tissue of the breast on a mammographic image. Mammographic density is a well-known risk factor for breast cancer [6]. However, few studies have been conducted to explore the association between mammographic density and hormone receptor status and molecular subtypes of breast cancer. Ours is one such study, in which we tried to observe if there is any association of mammographic breast density with hormone receptor status of breast cancer.
Although the patients in our study aged from 25 to 68 years, which was similar to those reported by previous studies [7, 8], the youngest patient in our study was younger than that seen in these studies. It was observed that 1% of breast cancer cases can be seen in young patients below 30 years of age [9]. We also observed that most patients had at least one living child and were postmenopausal, which was similar to previous studies [8,10].
Upon analysis of hormone receptor status, 41% of patients had ER-positive tumours, 33% of patients had PR-positive tumours, and 34% of patients had HER2-positive tumours. The frequency of ER-positive, PR-positive, and HER2-positive tumours in our study parallels previous studies [10], although the exact percentages vary, which may be attributed to the different study population and limited sample size of our study group.
The mean ages for ER-positive, PR-positive, and HER2-positive tumours in our study were close, being 47, 45, and 44 years respectively, which followed the general trend of ER+ and HER2+ receptor status seen in the older population compared to ER– and HER2– status, as mentioned in some studies [8,11]; however, the difference was not statistically significant.
Among morphological features, we noted that irregular masses with spiculated margins were common in ER-positive and HER2-positive patients, which was in concordance with previous studies [12,13]; however, this failed to reach statistical significance. The presence of calcification was significantly higher in HER2-positive tumours compared to HER2-negative tumours in our study, which resembled previous findings by some authors [12,13].
In our study, ER-positive tumours were more often seen in patients with high ABD compared to patients with low ABD, with mean ABD for ER+ and ER– tumours being 27.27% and 23.24%, respectively, and the difference was statistically significant. This was similar to the results stated in earlier research [14,15]. The absolute value for the mean ABD in our study differs from previous observations. This difference could be due to limited sample size, different ethnicity of our study population, and different software used to analyse ABD. However, no significant difference was seen in ABD of PR-positive vs. PR-negative tumours and HER2-positive vs. HER2-negative tumours, which is in agreement with previous research [16,17].
The strength of our study, by using automated software, is the decreased interobserver variability and reproducibility of assessed breast density [4], but it is limited by the small sample size and the lack of large-scale studies using Hologic Quantra software.
Conclusions
In our study we found a statistically significant difference between the mean ABD between ER+ and ER– tumours (p-value < 0.05). Ours is one of the first studies on an Indian population assessing the association of mammographic breast density with molecular subtypes of breast cancer, where the assessment has been done both visually and by computerised software
However, the study was done on Hologic Quantra computerised software version 2.1.1, which is a relatively new software, so further generalisation to other software can be erroneous. Some researchers studying different software have found that Quantra can overestimate and underestimate the dense volume and corresponding dense areas [18]. Also, Quantra version 2.1 does not specifically take into account the masking effect of local concentrated densities, which are governed by the distribution of parenchymal tissue and texture [3].
Hence, further, larger, multicentric studies and estimations from different software are required to assess this relationship, which can affect patient care.