Introduction
The current treatment outcomes for laryngeal squamous cell carcinoma (LSCC) remain moderate, and the 5-year survival rates are dependent on the anatomical location and the tumour-node-metastasis (TNM) staging score system [1]. Different types of therapy have improved outcomes, but distant metastases (DM) constitute a challenge in terms of prognosis [2]. Local metastases (LM) in lymph nodes and DM can develop after the curative first-line treatments, even if they were not present at initial diagnosis [3]. Therefore, oncologists want to know the metastatic potential of the initial tumour at diagnosis. While multiple genetic tumour biomarkers are being investigated for this purpose [4-6], advanced magnetic resonance (MR) techniques such as diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps may have the potential to predict LM and DM. Pre-treatment ADC and ADC alterations after the treatment have been shown to be correlated with the final response to the therapies, in previous papers [7-9].
The aim of this study was to evaluate the predictive value of ADC at initial diagnosis in treatment-naive patients with LSCC, for the development of future local and distant metastases.
Material and methods
Patient population
The local Ethics Committee approved this single-centre study and waived informed consent. Between October 2016 and January 2022, the hospital’s picture archiving and communication system (PACS) was retrospectively evaluated to identify treatment-naive adult patients with LSCC and MR examination including DWI. Inclusion criteria consisted of patients having biopsy-proven non-metastatic LSCC without previous treatment and planned curative surgical excision ± chemotherapy or chemotherapy ± radiotherapy. Patients without pathological diagnosis and follow-up imaging, patients with previous history of chemo-radiotherapy, patients with local lymph node or distant metastases at the time of the diagnosis, and patients having DWI with excessive artifacts that precluded drawing a region of interest (ROI) were excluded. The presence of LM and DM on follow-up was assessed by positron emission tomography (PET). LM means recurrence of the initial tumour at the neck lymph nodes after the treatment. DM means spread of the initial tumour to distant organs or distant lymph nodes.
MR imaging examinations
All MR examinations were performed by a 1.5 T MR system (Signa HDxt, GE Healthcare, Milwaukee, WI) with a 12-channel head-and-neck coil. The protocol included T1-weighted spin-echo (SE) before and after intravenous gadolinium-based contrast agent (repetition time ms/echo time ms, 683/10) and echo planar imaging (EPI)-DWI with TR/TE: 4571/70.5 ms; inversion time 160 ms, averages: 3; acquisition time ranged between 235 and 314 s; flip-angle: 90°; matrix size: 256 × 256, 21-28 slices. DWI images were obtained before the injection of gadolinium. ADC values were measured using 3 b-values (0, 50, and 800 s/mm2) (Table 1).
Table 1
Technical MR imaging parameters
| Parameter | Value |
|---|---|
| Sequence | DWI |
| Plane | Axial |
| TR/TE (ms) | 4571/70.5 |
| Averages | 3 |
| Acquisition time (s) | 235-314 |
| Flip angle (o) | 90 |
| Matrix size | 256 × 256 |
| Slice thickness (mm) | 5 |
| b-values | 0, 50, 800 |
| Number of slices | 21-28 |
In recent years, there have been indications for use in laryngeal cancer with b-values of 50 and 1000 [9-11]. On the other hand, it is worth noting that there are also studies in the literature that use b-values of 0 and 800 [12,13]. In this study, b-values 0, 50, and 800 were used due to its retrospective nature.
Image analysis
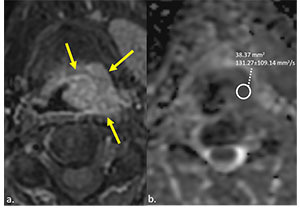
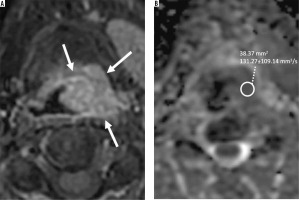
Delineation of the tumours was performed manually by 2 radiologists with 7 and 11 years of experience in head and neck radiology. Delineation was first made on post-contrast T1-weighted axial images and then on corresponding ADC maps (Figure 1). Only the solid tumour area, excluding cystic-necrotic components, was selected for analysis, and ADC calculations were made by 2 radiologists blinded to each other. ROIs were hand-drawn freely on the solid component of the tumour (Figure 1).
Figure 1
Post-contrast enhanced T1-weighted fat-supressed image on axial plane (A) shows supraglottic tumour (arrows) obstructing left piriform sinus in a 57-year-old man. Axial plane apparent diffusion coefficient (ADC) map (B) demonstrates diffusion restriction and low ADC value on the corresponding tumour region
Statistical analysis
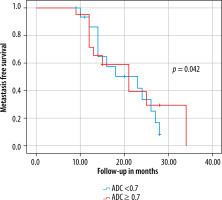
The relation between the presence of metastases and age, sex, ADC, initial primary tumour T stage, tumour localisation and volume were evaluated by univariate analysis and Student’s t-test. The inter-observer agreement for ADC measurements was assessed by intra-class correlation coefficient (ICC). An ICC > 0.75 indicates good agreement [14]. As previous studies emphasised, metastasis-free survival analysis regarding ADC < 0.7 × 10–3 mm2/s versus ADC ≥ 0.7 × 10–3 mm2/s was also assessed [15]. P-values < 0.05 were considered statistically significant. IBM SPSS Statistics, v. 21 for Windows (Armonk, NY) was used for all statistical analyses.
Results
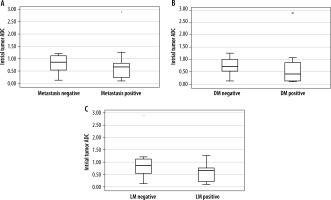
A total of 37 patients (32 males and 5 females) with a mean age of 62.8 ± 8.9 years (range 45 to 88 years) met the inclusion criteria and were enrolled in this study. Mean tumour volume and ADC were 4.8 ± 6.2 cm3 and 0.72 ± 0.51 × 10–3 mm2/s (range 0.10 to 1.89), respectively. T stages of the tumours before treatment were T1 (n = 5), T2 (n = 15), T3 (n = 11), and T4a (n = 6). Sixteen local lymph node and 8 distant metastases were detected on PET scans in a mean follow-up period of 17.5 ± 10.2 months. Both DM and LM were developed in 6 patients. A significant association between initial ADC and the presence of future distant metastases (p = 0.046; 95% confidence interval [CI] of the difference between –0.46 and 0.34) and future local metastases (p = 0.042; 95% CI of the difference between –0.71 and –0.13) was found. Mean ADC of the initial tumours that would develop distant and local metastases on follow-up, were 0.58 ± 0.23 × 10–3 mm2/s and 0.55 ± 0.34 × 10–3 mm2/s, respectively. The mean ADC of the initial tumours that would not develop distant or local metastases on follow-up was 0.94 ± 0.67 × 10–3 mm2/s. The difference of mean ADC values between future metastatic (0.57 ± 0.32 × 10–3 mm2/s) and non-metastatic (0.94 ± 0.67 × 10–3 mm2/s) initial tumours was significant (p = 0.017) (Table 2, Figure 2). When the relationship between the patient demographics, tumour variables, and the status of metastases were assessed, besides ADC, only the tumour volume was found to be significantly associated with the presence of metastases in this series (p < 0.001) (Table 2). The inter-observer agreement was high, as ICC = 0.91 [95% CI: 0.86-0.95]. Metastasis-free survival curves according to ADC are shown in Figure 3.
Table 2
The association between patient demographics, ADC, tumour variables, and the presence of metastasis.
Discussion
The current study demonstrated that pre-treatment ADC values could be used to predict future local or distant metastases in patients with treatment-naive LSCC. In other words, the metastatic potential of the initial LSCC at diagnosis might be anticipated via ADC maps. Driessen et al. [12] found the ADC of the laryngeal or hypopharyngeal SCC ranging between 0.92 and 1.30 × 10–3 mm2/s in their study involving 16 patients. Although the ADC of the tumours in this series also varied widely, we have demonstrated that the mean ADC values of the future metastatic LSCCs showed a significant difference to those with no future metastases. Similarly to our findings, Brenet et al. [15] stated that an initial ADC below 0.7 was significantly more common in patients with residual head and neck (HN) SCC after chemoradiotherapy. They found an mean initial ADC of the residual tumours of 0.56 ± 0.11 × 10–3 mm2/s. Parallelly, we have found a mean initial ADC of future distant and local metastatic tumours of 0.58 ± 0.23 × 10–3 mm2/s and 0.55 ± 0.34 × 10–3 mm2/s, respectively. They also highlighted that ADC elevation at 3-month follow-up MR lower than 0.7 was associated with disease progression or recurrence. Initial ADC < 0.7 (n = 14) was more frequent than ADC ≥ 0.7 (n = 4) in patients with future metastatic LSCC in this series, and metastasis-free survival was significantly better for patients with initial ADC ≥ 0.7 compared to ADC < 0.7 (Figure 3). Some authors stated that lower ADC of initial HN tumours significantly correlated with the persistent or recurrent HNSCC [16]. In contrast to our results, King et al. [17] stated that initial ADC showed no correlation with local failure in patients with HNSCC. Although they found the mean ADC of the tumours with local treatment failure to be lower than those with local control (1.45 ± 0.27 × 10–3 mm2/s versus 1.62 ± 0.29 × 10–3 mm2/s, respectively), this difference was not significant. This discrepancy of results might be due to the heterogeneous sample of their study including primary tumour sites of the oral cavity, oropharynx, hypopharynx, larynx, nasal cavity, and maxillary sinus. Conversely, the current study consisted of patients with only LSCC. Lombardi et al. [18] showed that pre-treatment ADC of patients with local control for HNSCC was lower than that of patients with persistent or recurrent disease. Their study again harboured a heterogeneous tumour group of 47 patients with HNSCC. In their study, 42 patients had nasopharyngeal and oropharyngeal carcinomas, 5 hypopharyngeal carcinomas, and there was no laryngeal SCC. Furthermore, in contrast to our ROI drawing, they selected the ROI for the whole tumour region including cystic/necrotic components.
Several papers have shown the utilisation of ADC changes before and during, or before and after, chemoradiotherapy to anticipate local tumour recurrence in HN cancers [19-23]. However, those studies contained mostly extra-laryngeal SCCs (including oropharyngeal, hypopharyngeal, nasopharyngeal, paranasal sinuses, oral cavity tumours, and metastatic neck lymph nodes), and the data of ADC measurements originated from this large heterogeneous primary tumour location. Tomita et al. [24] demonstrated a deep learning method for outcome prediction in patients with laryngeal and hypopharyngeal cancers, and they found that a lower intratreatment DWI signal was significantly associated with better 2-year progression-free survival. They used a deep learning approach to eliminate the potential variabilities in ADC calculations including the shape and site of the ROI, and low reproducibility and objectivity. Matoba et al. [21] showed that pretreatment ADC was not statistically correlated with the local tumour control, in contrast to the early treatment ADC alterations. Nevertheless, all those papers emphasised, and aimed to predict, local tumour control with ADC measurements. Conversely, we aimed to predict the potential future local and distant metastases by calculating the initial tumour ADC, and we demonstrated a significant difference between the ADC of the future metastatic LSCCs over those with no future metastases. In addition to ADC, pre-treatment tumour volume was the other variable that was significantly associated with the presence of future metastases. Sistonen et al. [25] found that tumours larger than 7 cm3 had increased locoregional recurrence, in a retrospective study harbouring 67 patients with oropharyngeal SCC. Similarly, mean tumour volume was 8.2 ± 7.2 cm3 in the metastasis-positive group in this series.
There were several limitations in the current study. Firstly, the retrospective design of the study was the main restriction. Secondly, the patient population was limited, and the follow-up period was relatively short. However, despite the small sample size, the pathology of the tumours were all LSCC and homogeneous compared to the heterogeneous study populations of the previous papers including whole HN tumours. Finally, pathological parameters such as tumour stroma, and cellular or nuclear density of the tumour were not evaluated in this study.
Conclusions
Pretreatment volume and ADC values of the initial tumour showed the ability to predict the development of future local or distant metastases in patients with LSCC in this series. Therefore, diffusion-weighted MR can be used as an imaging modality to differentiate patients with high potential for the development of local and distant metastases.
Disclosures
1. Institutional review board statement: The study was approved by the Institutional Ethics Committee of the Umraniye Training and Research Hospital, with approval number: B.10.1.TKH.4.34.H.GP.0.01/348.
2. Assistance with the article: None.
3. Financial support and sponsorship: None.
4. Conflicts of interest: None.