Introduction
In imaging, a hepatic abscess (HA) can present variously. Radiological features are associated with the morphological stage of HA formation. In the early stages, it can be easily misdiagnosed as a neoplasm or metastasis, and in the late phase of the disease, it may resemble a cyst [1-3]. Color Doppler can also be misleading when assessing the vascularization of focal lesions, as this can vary depending on their morphological stage. This is particularly true when differentiating hepatocellular carcinoma from metastasis, which can appear similar to an HA on imaging. Additionally, color Doppler shows insufficient specificity, especially in cases of fatty liver. Furthermore, hepatocellular carcinoma may originate from a hepatocellular adenoma that has progressed. Hepatocellular adenomas can also have hemorrhagic foci, which can resemble a malignant process despite the lack of malignancy [4-7]. It is expected that the abscess will contain purulent, hypoechogenic fluid in the center; however, liquefaction can develop even 2 weeks later [8-10]. B-mode ultrasonography (B-mode) and computed tomography (CT) are reported to be sufficient in diagnosing more than 90% of HA cases. It is reported that the sensitivity of tri-phasic enhanced multi-slice CT has an advantage in this aspect over B-mode [11]. Furthermore, the visualization of HA in B-mode can vary, correlating with the type of pathogen causing the infection, the immunocompetency of the patient, and the stage of the disease. The specifics of the disease, associated with variable stages of HA morphology as the disease progresses, and the necessity of monitoring the effects of treatment, require frequent repetition of imaging. B-mode can be insufficient in this aspect, while contrast-enhanced CT exposes the patient to radiation and can be problematic in patients with renal failure. Contrast-enhanced ultrasound (CEUS) appears to enable the acquisition of images of abscesses with similar diagnostic value, avoiding the issues mentioned earlier. The study aimed to analyze the diagnostic value of CEUS compared to B-mode and assess the potential utility of this imaging modality in diagnosing and monitoring the HA treatment process.
Material and methods
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Bioethics Committee (RNN/266/22/KE). All patients provided informed consent to participate in the project. The inclusion criteria involved the confirmation of an HA by contrast-enhanced CT and CEUS within 48 hours before or after CT (usually with approximately a 24-hour interval). Consideration was given to the presence of one or more of the following clinical symptoms: pain in the right upper quadrant, jaundice, tenderness, fever, nausea, weight loss, or night sweats. Additionally, elevation of one or more specified laboratory parameters such as leukocytosis, CRP, procalcitonin, ALT, and AST was considered. The exclusion criteria were in line with the contraindications to using contrast in ultrasonography according to the recommendation of the SonoVue producer, which included respiratory insufficiency, acute coronary syndrome, adverse post-contrast reactions, or declaration of pregnancy.
Patients
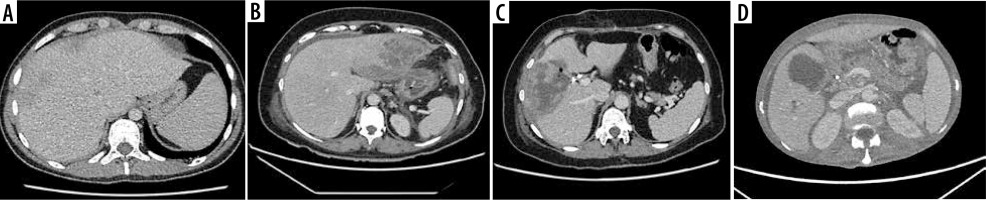
The retrospective study involved 29 patients diagnosed with HA, 9 of whom had multiple lesions (> 2). The study included CT scans, and the obtained images were compared with B-mode and CEUS. Both procedures were performed within a time interval of less than 48 hours. GE Healthcare Revolution CT and GE Lightspeed VCT 64 Slice scanners were utilized. The CT examinations were conducted in accordance with LI-RADS Version 2018 [12], considering post-contrast sequences with assessment of late enhancement (1 ml of Ultravist-370 per kg of body weight). The size of the lesions was evaluated early after contrast administration. In addition, the abscess was classified into one of the four morphological forms described by Giampiero Francica: I – tumor-like with no or little liquified component; II and III – honeycomb type, with the intermediate stages reflecting the progression of liquefaction of the inflamed residual parenchyma inside the abscessed cavity; IV – cystic-like [13]. Demographic information and details of the studies conducted are presented in Table 1.
Imaging technique
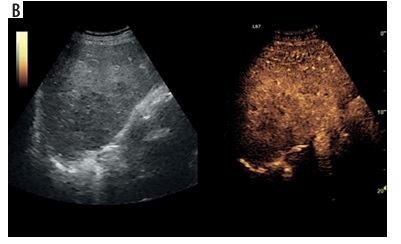
CEUS was conducted in accordance with the 2020 update of the guidelines for CEUS in the liver [14]. A GE Logiq 7 system equipped with a convex probe 4C was utilized for the procedure. Initially, a B-mode ultrasound examination of the liver was performed to record the size, localization, and quantity of lesions. Following this, color Doppler imaging was executed to confirm the lack of vascularization within the HA. The final step involved performing CEUS, which began with an injection of 2.4 ml of the contrast agent SonoVue into the medial cubital vein. This amount typically sufficed to achieve an accurate diagnosis, though additional contrast agent was administered if necessary, particularly in cases involving multiple lesions. The CEUS was carried out using a low mechanical index (< 0.1) to prevent the destruction of the contrast agent bubbles [15,16]. The examination included three main acquisition phases with specified intervals: the arterial phase (10-45 seconds), the portal venous phase (45-120 seconds), and the late venous phase (120-180 seconds) [15]. No significant dynamic enhancement profile changes were observed after 120 seconds. Additionally, lesions situated deep under the diaphragm required deep inhalation and breath-holding, which posed difficulties for some patients and made extended acquisition challenging. Extended acquisition was only recorded in ambiguous cases. The enhancement of the HA walls and purulent regions was compared to the liver parenchyma (Figure 1). A key parameter of the CEUS examination was the assessment of the HA capsule enhancement and the lack of enhancement in the fluid component throughout the entire examination period, regardless of the enhancement of the HA capsule and liver parenchyma. CT imaging served as the reference standard, and the assessment of the HA morphological form adhered to the methodology described by Francica [13]. Figure 2A-D illustrates the consecutive stages of disease development according to this methodology. The assessment and classification of obtained images were performed in consensus by 2 radiologists. In instances where changes at the borderline of morphological stages were uncertain, the higher morphological stage was selected.
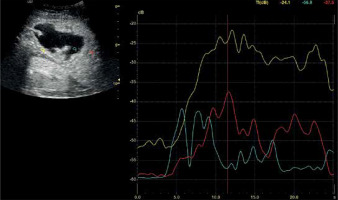
Figure 1
Assessment of the degree of enhancement in the post-contrast examination (CEUS) involved placing regions of interest (ROIs) within the lesion (blue for pus, yellow for abscess capsule) and another area in the liver parenchyma (red). Enhancement curves were recorded for 2-3 minutes in sequences of approximately 20-30 seconds each. Values affected by motor artifacts causing the displacement of the areas of interest were eliminated
Statistical analysis
A statistical analysis was conducted on demographic data (age, sex) and the sizes of both smaller and larger dimensions of lesions, and the morphological type of HA was also defined. Echogenicity of the marked regions was acquired in the arterial phase, portal venous phase, and late venous phase. The distributional characteristics of the echogenicity in each phase are presented using a boxplot, where the median value is marked by the line in the center of the box. No outliers were observed in the collected data. The Python Matplotlib v3.6 package was employed to create the plot, and the SciPy Python library was used for quantitative statistical analysis. The size of the abscesses in the three imaging methods (CT, CEUS, B-mode) was compared, and the difference between pus/liver parenchyma and pus/abscess pouch echogenicity was assessed using a statistical test. Due to non-normal distribution and a small sample size, a non-parametric two-sided Mann-Whitney U test was used to check the statistical power of the mentioned parameters. Drainage is considered the gold standard for the treatment of abscesses, although for small lesions < 4 cm it is suggested that conservative treatment may be sufficient. Therefore, in addition to performing calculations for the entire study group, the group was also subdivided into lesions > 4 cm and < 4 cm to determine whether there were differences in CEUS examination depending on lesion size [17]. A p-value less than 0.05 was considered statistically significant. Concordance between CT/CEUS and CT/B-mode is presented in Table 3. The provided data include the number of true positives, true negatives, false positives, and false negatives for CEUS and B-mode assessments compared to the gold standard (CT), with an estimated sensitivity ranging from 84.6% to 97% and a specificity of around 97% [18]. According to the methodology adopted from Francica’s study, tumor-like lesions were considered abscesses and included in the study only after a diagnostic puncture of the lesion. Percent agreement and Cohen’s κ were also calculated, following the method described by McHugh et al. [19]: values ≤ 0 – no agreement, 0.01-0.20 – no to slight agreement, 0.21-0.40 – fair agreement, 0.41-0.60 – moderate agreement, 0.61-0.80 – substantial agreement, and 0.81-1.00 – perfect agreement.
Table 2
Dimensions of lesions in CEUS, CT, B-mode
Table 3
Agreement between ultrasound methods and computed tomography (CT)
Results
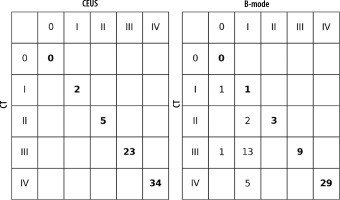
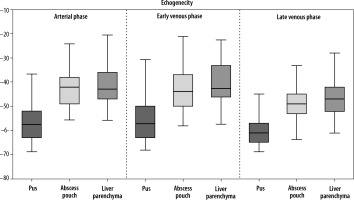
Estimated lesion sizes based on post-contrast sequences on CT, post-contrast sequences on CEUS, and B-mode studies, along with statistically calculated differences, are shown in Table 2. Ultrasonographic images obtained without and with contrast enhancement were compared to contrast-enhanced CT used as a benchmark. Based on CT scans, the following numbers of lesions were classified as individual morphological forms: I – 2, II – 5, III – 23, IV – 34. The classification of lesions on CEUS showed full concordance with those on CT. In B-mode, compared to CT, 2 lesions of type I were not detected; the rest of the HA were categorized as follows: I – 21, II – 3, III – 10, IV – 30 (Figures 3, 4A-D, 5A-D, 6A-D and 7A-D). The percent agreement and Cohen’s κ between ultrasound methods and CT were calculated for type III and IV HA; due to the small sample size for groups I and II, they were not calculated (Table 3). The enhancement values of pus and the abscess pouch were measured and compared with the liver parenchyma in the arterial, portal, and late venous phases. Pus enhancement values remained similar during the examination (pus background gain difference was ± 15 dB). The values of the abscess pouch background difference changed from ± 11 dB in the arterial phase to ± 6 dB in the late venous phase (Figure 8). The Mann-Whitney U test values for confirmation of these observations were calculated in every phase. For the entire study group: arterial phase – pus vs. abscess pouch difference (U = 451.0, p = 1.02e-14), pus vs. liver parenchyma (U = 378.0, p = 6.68e-16), portal phase – pus vs. abscess pouch difference (U = 621.0, p = 3.79e-12), pus vs. liver parenchyma (U = 402.0, p = 1.61e-15), late venous phase – pus vs. abscess pouch difference (U = 558.5, p = 4.53e-13), pus vs. liver parenchyma (U = 334.0, p = 1.18e-16). There was no statistically significant difference in the pus and abscess pouch enhancement values on examination depending on the size of the abscess (> 4 cm group vs. < 4 cm): arterial phase – pus (U = 509.5, p = 0.9155), abscess pouch (U = 739.0, p = 0.0034), portal phase – pus (U = 420.5, p = 0.1983), abscess pouch (U = 595.0, p = 0.3105), late venous phase – pus (U = 539.0, p = 0.7855), abscess pouch (U = 645.0, p = 0.0932). During the analysis of multiple examinations in the process of monitoring the disease course, a trend of abscess reclassification to both higher and lower stages was observed. In cases where transition to a higher stage occurred, it was associated with disease progression, whereas in cases of transition to a lower stage, it was most likely related to the treatment applied.
Figure 4
A) Ultrasound and color Doppler: transverse view shows focal lesion in right liver lobe, identified as type I abscess via CT. Lesion echogenicity slightly lower than liver. B) CEUS arterial phase: abscess intensity higher than liver, with inhomogeneous enhancement. C) CEUS portal phase: abscess intensity remains higher than liver, with inhomogeneous enhancement and wash-out effect. D) CEUS late venous phase: abscess intensity remains elevated compared to liver, with inhomogeneous enhancement and continued wash-out effect
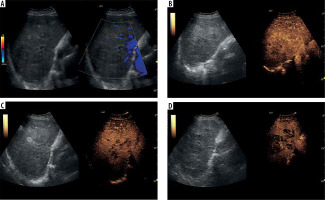
Figure 5
A) Ultrasound and color Doppler: transverse view shows focal lesion in left liver lobe, classified as type II abscess via CT. Lesion echogenicity slightly lower than liver, with obscured liquid part. B) CEUS arterial phase: Abscess intensity slightly higher than liver, with inhomogeneous enhancement; liquid component visible. C) CEUS portal phase: abscess intensity slightly lower than liver, with inhomogeneous enhancement and wash-out effect. D) CEUS late venous phase: abscess intensity maintains slight decrease compared to liver, with inhomogeneous enhancement and continued wash-out effect; type II pattern confirmed with visible liquid component throughout phases
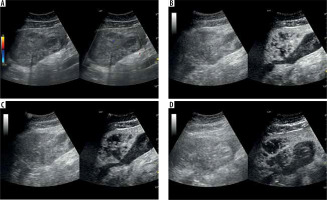
Figure 6
A) Ultrasound and color Doppler: transverse view shows focal lesion in right liver lobe, classified as type III abscess via CT. Lesion echogenicity slightly lower than liver, no visible liquid part. B) CEUS arterial phase: abscess capsule clearly visible, undergoing intense contrast enhancement; fluid component visible. C) CEUS portal phase: wash-out effect visible from abscess sac, fluid component unchanged. D) CEUS late venous phase: further washout effect visible; abscess capsule enhancement less intensive compared to liver parenchyma; type III pattern confirmed with visible fluid component throughout phases
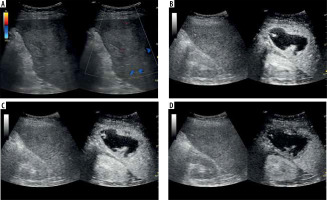
Figure 7
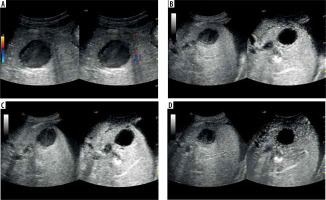
A) Ultrasound and color Doppler: transverse view shows focal lesion in right liver lobe, classified as type IV abscess via CT. Lesion echogenicity lower than liver, visible liquid part and zone of edema. B) CEUS arterial phase: abscess capsule clearly visible, undergoing intense contrast enhancement. C) CEUS portal phase: wash-out effect visible from abscess capsule, fluid component unchanged. D) CEUS late venous phase: further wash-out effect visible; abscess capsule enhancement less intensive compared to liver parenchyma; type IV pattern confirmed with visible liquid component throughout phases
Discussion
HA is an inflammatory focal liver lesion. Procedures within the biliary tract are important risk factors associated with a higher risk of HA occurrence. Clinically, HA is highly nonspecific; the most common symptoms reported by patients are pain in the right upper quadrant, jaundice, tenderness, fever, nausea, weight loss, and night sweats. In laboratory testing, leukocytosis, elevated inflammatory parameters, and liver enzymes can be observed [16,17, 20-23].In the past, HA were treated by surgical drainage and antibiotics; nonetheless, they were associated with a high mortality rate. Today, advancements in interventional radiology enable the performance of percutaneous drainage with antibiotic therapy, significantly reducing the number of complications and the mortality rate. Furthermore, convalescence after percutaneous intervention is considerably shorter compared to surgery [8,24,25]. Surgical drainage can be employed as a first-line treatment in cases of multiple or percutaneously inaccessible abscesses, accompanying peritonitis, abscess rupture, or when there is additional need for biliary tract surgery. Surgical drainage can also be chosen as a last-line treatment when less invasive procedures fail [17,23] In imaging, HA is highly nonspecific. The “double target sign” is considered a pathognomonic radiological symptom in CT. It consists of a hypodense central zone of purulent fluid; additionally, gas bubbles can be observed, and the inner zone is represented by a pyogenic membrane that exhibits contrast agents during the arterial phase. The outer zone is caused by edema of surrounding liver parenchyma [26]. The most common image of HA in B-mode is a hypoechoic mass with the potential presence of septa and gas bubbles, surrounded by a thick, irregular wall. In the early phase of formation, HA appears as a solid homogeneous mass, which can be problematic in differentiation from liver solid tumors. In the later phase, liquefaction causes changes in homogeneity, and the lesions start to become inhomogeneous. Lastly, when the HA is fully formed, the central hypoechogenic region of fluid surrounded by a thick wall is visible, resembling a cystic lesion [22,24,27]. This may raise doubts about the next diagnostic and therapeutic steps. It may result in performing a liver biopsy in the case of suspected cancer (form I) instead of drainage, or placing the drain outside the fluid component of the lesion (forms II and III), which would result in ineffective drainage and the need to relocate the drain or repeat the procedure. In CEUS, the following features commonly characterize HA: marginal rim and septa enhancement (honeycomb appearance), lack of enhancement in central liquified parts, and wash-out of the enhancing part of the lesion in the later part of examination [9,22,24]. Nonetheless, the radiological image of HA depends on the disease’s progression. Giampiero Francica identified 4 sonomorphological stages of the disease: I – tumor-like with no or little liquified component; II and III – honeycomb type, the intermediate stages reflecting the progression of liquefaction of the inflamed residual parenchyma inside the abscessed cavity; IV – cystic-like [13]. Other authors reported that B-mode was insufficient in distinguishing between the stages of the disease; furthermore, 4/113 abscesses were completely invisible in the examination. In CEUS, the characteristic features of abscesses were detected: subsegmental/segmental hyperemia 93/113, necrosis with hyperemic margin 109/113, the wash-out effect 101/113 [20]. Our observations seem to confirm that the sensitivity of CEUS is higher than B-mode. Two lesions classified as tumor-like were not visible in this method, whereas CEUS was able to visualize them. Furthermore, in B-mode, the liquefied component of the HA was not visible in 21/64 cases, which could lead to the classification of the lesion as a solid mass. In the case of type II and III morphological forms, the entire liquid part was not visible, leading to the incorrect classification of 2/5 type II and 13/23 type III HA. In the case of the type IV form, 4/34 lesions were not correctly classified. Popescu et al. reported that the CEUS was conclusive in 38/41 cases in their study. Every conclusive case presented necrosis with a hyperemic margin (38/41); subsegmental/segmental hyperemia was observed in 17/41 cases, while the wash-out effect was presented by 22/41 lesions (22). Giampiero Francica, in his study, reported that a hyperemic margin was observed in 38/44 cases, liquified necrotic areas in 39/44, and subsegmental/segmental hyperemia in 40/44 cases. The wash-out effect occurred in 30/38 cases [13]. It is worth mentioning that some authors reported that the clinical condition of the patient and the results of laboratory testing can be associated with the morphology of the abscess [28]. The results presented in Table 3 suggest that, while B-mode efficiency is high for cystic-like HA, CEUS appears to be better at visualizing the fluid component of the lesion in their earlier stages. However, due to the small number of type I and II lesions, further studies with a larger sample size are needed to verify this hypothesis. Furthermore, we noted that none of the authors considered the presence of gas bubbles inside the abscess in the classification, which may lead to its misclassification into one of the subgroups. These days, percutaneous needle aspiration or percutaneous catheter drainage, guided by ultrasound combined with antibiotics, is considered the primary method of treating HA [23,25,29]. Furthermore, we would like to emphasize that during treatment at our institution and follow-up examinations after successful treatment, we observed regression in the morphological stage of the HA. This observation underscores that the morphological classification of HA can vary and may not definitively predict the course of the disease. While the authors agree on the preferred method of HA treatment, the selection of lesions suitable for surgical drainage or those eligible for less invasive percutaneous intervention, the necessity of radiological monitoring during treatment, and the potential timing for catheter removal are widely discussed, with no specific guidelines provided in this aspect. Wadhera et al. [17] opined that antibiotic therapy can be sufficient to manage patients with small HA < 3/4 cm, while HA > 5 cm should be treated with drainage. Our measurements of HA using all three methods discussed above support the hypothesis that CEUS, in addition to assessing the morphological type of the HA, is as effective as CT in estimating its size. B-mode, on the other hand, with respect to both CT and CEUS, appears to be a method that underestimates the size of the lesion. This observation, however, did not show the expected level of statistical significance, so a larger study group is required to test this hypothesis. If this observation were confirmed in a larger study group, as proposed by Wadhera et al., it could result in incorrect decisions regarding the selection of patients for drainage procedures using only B-mode. Moreover, as mentioned above, it is reported that the liquefied component of HA can be present; however, it does not have to be visible in B-mode. Our analysis of the enhancement values of the pus and the abscess capsule, and their comparison in the three phases of the contrast examination, indicates a real tendency towards a wash-out effect, although this phenomenon is not consistent across all cases. Nevertheless, a significant difference in enhancement between the pus, the abscess capsule, and the liver parenchyma during all phases of the examination was observed. Furthermore, dividing the abscesses into a group < 4 cm, which may potentially be treated conservatively, and a larger group > 4 cm, where interventional treatment should be considered, and analyzing the enhancement values within these groups in all phases, indicates that there is no significant difference between the subgroups. This suggests that CEUS will be a suitable tool for the evaluation of HA regardless of size. Some authors have suggested that CEUS may be useful during interventional radiology procedures. For example, it facilitates adequate tissue sampling during biopsy of hepatocellular carcinoma, which is characterized by the presence of necrosis or bleeding inside the lesion. Conducting a biopsy under the guidance of CEUS enables visualization of the appropriate area for the removal of material with suitable diagnostic value [6,30]. CEUS can also be employed to determine the optimal site for abscess drainage and to monitor its progression over time. In our opinion, CEUS can be effectively utilized to identify HA eligible for drainage, determine the optimal location for drain placement, and monitor the treatment’s progress. As mentioned earlier, the enhancement values of the liquid component of the abscess remain consistent throughout all phases of the CEUS examination, enabling a comprehensive examination of the entire liver parenchyma for abscess foci without time constraints. This pressure is typically present in cases of liver tumors, where diagnosis strongly relies on capturing the nature of contrast influx into the lesion during the short-lived arterial phase (10-45 seconds) [6]. Hence, CEUS serves as a valuable method for monitoring in such cases. However, in the case of HA, it appears to be a promising method for both diagnosis and monitoring. In our opinion, CEUS not only aids in diagnosis but also can be used as an evaluative tool for patients undergoing percutaneous intervention, enhancing the effectiveness and safety of invasive procedures through precise guidance. We plan to conduct further research on the application of CEUS, with a particular focus on HA drainage. Special consideration will be given to changes in the liquid component during the procedure. In our opinion, CEUS provides fully sufficient diagnostic accuracy and is a safe method that does not expose the patient to radiation. Furthermore, it can be used in patients suffering from renal failure, as the contrast agent is eliminated through the airways [14].
Conclusions
CEUS is a valuable imaging modality for diagnosing and monitoring HA compared to B-mode. The purulent component in B-mode is not always visible. During the CEUS examination, the abscess pouch, septa, and liver parenchyma are more clearly visible than in B-mode. The purulent part of the lesion does not exhibit contrast agents during all phases of the examination. This allows the physician to perform an accurate assessment of the organ without time pressure, offering several advantages over traditional methods, and making it a preferable option in clinical practice. Therefore, in our opinion, CEUS can replace CT in monitoring the disease. Moreover, CEUS seems to be a promising tool in assessing patients’ eligibility for percutaneous intervention.
Disclosures
Institutional review board statement: The study was approved by the Ethics Committee of the Medical University of Lodz, approval number: RNN/266/22/KE.
Assistance with the article: None.
Financial support and sponsorship: The research was funded from the statutory fund of the department under grant number 503/1-136-01/503-11-001.
Conflicts of interest: None.