Introduction
Pulmonary embolism most commonly refers to obstruction of the pulmonary arterial vasculature by thrombus secon-dary to venous thrombo-embolism [1]. It is a potentially life-threatening illness, which is commonly diagnosed in an emergency setting and leads to increased morbidity and mortality [2,3]. Since the clinical application of multidetector computed tomography (MDCT), computed tomography pulmonary angiography (CTPA) is now established as a modality of choice for diagnosis of patients suspected of acute pulmonary embolism (APE) [4-6]. However, diagnosis of subsegmental pulmonary embolism (SSPE) remains a challenge [7-12].
Pulmonary arteries and their branches are influenced by cardiac and respiratory motions, which lead to blurring of broncho-vascular structures and shading artefacts in contrast-filled arteries, and they degrade the image quality of lung parenchyma affecting the detection of SSPE [13-15]. With currently available MDCT/dual-source computed tomography (DSCT), respiratory motion-related artefacts have been addressed due to faster scanning and large area coverage [16,17]. Cardiac motion related artefacts have been studied by electrocardiogram (ECG)-gated CTPA with improved image quality [18,19]; however, it was associated with increased radiation exposure due to retrospective ECG-gated data acquisition, which acquires data continuously throughout the cardiac cycle [5,20]. However, with application of prospective ECG-gated pulmonary angiography radiation exposure, the concerns were largely addressed [14,15,21,22]. However, these studies were limited to relatively stable patients and the authors did not find any published study on the application of high-pitch prospective ECG-gated (HP-PECG-gated) CTPA for the diagnosis of SSPE in patients suspected of APE. In this context we conducted a prospectively designed randomized study on second-generation 128-DSCT on patients clinically suspected for APE with HP-PECG-gated CTPA and standard-pitch non-ECG-gated (SP-NECG-gated) CTPA for the detection of SSPE. In addition, cardiac motion-related artefacts, image quality, and quantitative parameters: pulmonary arterial enhancement, radiation exposure, contrast volume, and scan time, were also compared.
Material and methods
This randomized prospective study was conducted in a fede-rally funded non-profit tertiary care institution from January 2015 to June 2016. A total of 87 patients were enrolled in the study, who had clinical suspicion of APE. The study protocol was approved by the institutional Ethics Committee, and informed written consent was obtained from all the patients.
Exclusion criteria: those with presence of a pacemaker, body mass index (BMI) over 40 kg/m2, cardiac arrhythmia, known significant lung parenchymal disease, pregnancy, and contraindication to intravenous iodinated contrast such as predisposition for contrast reaction or deranged renal function tests were excluded from the study.
The patients who fulfilled the inclusion criteria were randomly assigned by random integer generator with a list of 1s and 2s assigned to either HP-PECG-gated CTPA (n = 44) or SP-NECG-gated CTPA (n = 43) on second-generation 128-DSCT (SOMATOM sensation-definition-flash; Siemens Healthcare, Erlangen, Germany).
Scanning protocols
Area coverage: The scan range for both the groups extended from lung apices to the dome of the diaphragm with scanning in the cranio-caudal direction.
Contrast injection protocol: For both groups contrast was injected through the anterior cubital vein via a dual syringe power injector with a flow rate of 5 ml/s. Saline chase at the same rate was given in high-pitch protocol for 10 s. Bolus tracking technique was used for acquisition of data with region of interest (ROI) placed in the main pulmonary artery (MPA) in both the groups with automatic scanning after a 5-second delay once a preset attenuation value of 100 was achieved in MPA.
Contrast volume: Vascular opacification was achieved by injecting non-ionic iodinated contrast (Omnipaque 300, GE Healthcare, Ireland) in both the groups. Contrast volume calculation for both groups was as follows [flow rate × (scan time + scan delay)]. In addition, 10-15 ml was added in both groups to achieve partial opacification of left-sided cardiac chambers and aorta.
Scanner details: Second-generation 128-DSCT (SOMATOM sensation-definition-flash; Siemens Healthcare) with temporal resolution of 75 milliseconds, gantry rotation time of 0.28 seconds, and slice/beam thickness of 0.6 mm.
Radiation optimization techniques: Tube voltage 100 kVp (after switching off Automatic Care kV), automated tube current modulation (care dose 4D; Siemens health care), and iterative image reconstruction algorithms.
Pitch for HP-ECG-gated CTPA was 3.2, while for SP-NECG-gated CTPA it was 1.2.
HP-PECG-gated CTPA was prospective ECG-gated acquisition with system-generated single dataset in diastolic phase of cardiac cycle prefixed between 60 and 80% of the R-R interval of the ECG depending upon the heart rate. For image reconstruction the iterative reconstruction (IR) method was used to provide good quality images.
Image analysis
Images were reconstructed to 1 mm slice thickness and analysed on a dedicated workstation (Syngo.via, Siemens Healthcare, Germany). Pulmonary embolism was diagnosed as hypodense filling defect/s in the pulmonary vasculature as established criteria [9,13].
The presence of SSPE and image quality were analysed by 2 radiologists (MS, UG) blinded to each other’s findings, and interobserver agreement was calculated for each finding.
Qualitative analysis of images
Images were scored on Likert scales for various artefacts as per the previously published articles, as follows [8,9]:
Blurring of broncho-vascular structures – severe: 1 – indistinct edges within discernible structures, moderate, 2 – significantly blurred edges but discernible, minor, 3 – minimal blurring, and none, 4 – clearly discernible edges with no blurring.
Double-line artifact – severe: 1 – clearly discernible doubling of edges and if associated with many structures, moderate, 2 – fairly discernible doubling of edges and associated with several structures, minor, 3 – barely discernible doubling of edges and seen only in one or two structures and, none, 4 – no blurring of edges.
Intravascular shading – severe: 1 – marked and widespread darkening in opacified pulmonary arteries, mode-rate, 2 – patchy and discernible darkening, 3 – localized and barely discernible darkening and, 4 – uniform opacification with no darkening.
Lung image quality – 1 – artefacts affecting diagnostic assessment, 2 – artefacts limiting the visibility of major structures but still diagnostic, 3 – minor artefacts, and 4 – no artefacts.
For each Likert scale, scores of 1 and 2 were considered as non-diagnostic. Scores of 3 and 4 were considered diagnostically acceptable.
Quantitative analysis of images
Pulmonary arterial enhancement: measurement by placing the ROI to obtain a value in Hounsfield units (HU) in the main, left, and right pulmonary arteries in each patient.
Contrast volume: noted in millilitres (ml) for every patient in both groups.
Acquisition time: system-calculated acquisition time was recorded in each patient.
Radiation exposure: system-generated radiation exposure dose length product (DLP in milligray centimeters – mGy cm) was recorded for each patient, and effective radiation dose (millisieverts – mSv) was calculated by using the appropriate conversion factor (0.014) as per ICRP 103 publication recommendations [23].
Statistical analysis
The statistical analysis was done using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 22.0 for Windows). Means and medians were calculated for quantitative variables, and for measures of dispersion standard deviations or interquartile ranges were calculated. Normality of data was checked by measures of Kolmogorov-Smirnov tests of normality. For normally distributed data, means of 2 different groups was compared using the t-test. For skewed data and ordinal data the Mann-Whitney test was applied. All statistical tests were 2-sided and were performed at a significance level of a = 0.05. For the reliability between 2 observer findings Cohen’s κ test was used and the κ value derived.
Results
A total of 87 (n = 87) patients were enrolled in the study with 44/87 subjected to HP-PECG-gated CTPA (n = 44) and 43/87 to SP-NECG-gated CTPA (n = 43). Patient variables and characteristics were comparable without any statically difference and are summarized in Table 1.
Table 1
Patients’ demographics
Location of pulmonary embolism
Pulmonary embolism was diagnosed in 15/44 (34.09%) patients in HP-PECG-gated CTPA and in 10/43 (23.26%) patients in standard SP-NECG-gated CTPA (Figure 1). In diagnosed cases, thrombus was present at various levels of pulmonary vasculature and summarized in Table 2. SSPE was present in 15/44 (34.09%) patients in HP-PECG-gated CTPA, in comparison to 8/43 (18.60%) patients in SP-NECG-gated CTPA (Figures 1 and 2).
Figure 1
High-pitch PECG-gated CTPA in a 24-year-old female patient – coronal and axial images show hypodense filling defect in one of the subsegmental branches of the left inferior pulmonary artery (arrow in A) and in a segmental branch of the right inferior pulmonary artery (arrow in B). Note clearly discernible segmental and subsegmental branches of pulmonary arteries (score 4) devoid of double-line artefacts and intravascular shading (score 4)
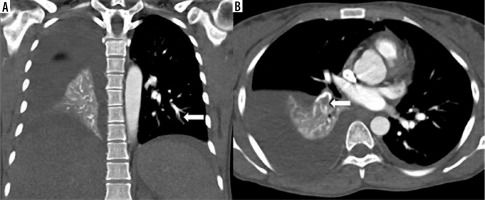
Figure 2
High-pitch PECG CTPA in an 18-year-old male patient shows a hypodense filling defect in one of the subsegmental branches of the left inferior pulmonary artery (arrow). Segmental and subsegmental branches of pulmonary arteries are free of motion blur and of double-line artefacts (score 4) however, minor intravascular shading is present (score 3)
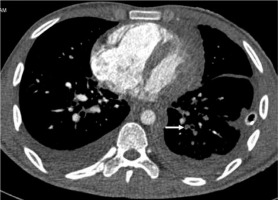
Table 2
Locations of thrombus in pulmonary vasculature
Qualitative measurements of image quality
Statically significant less blurring of bronchovascular structures and double-line artefacts with p-value less than 0.001 and 0.01, respectively, were noted in HP-PECG-gated CTPA (Figure 3), while intravascular shading was comparable (p = 0.15). The scores of each of these parameters are detailed in Table 3.
Figure 3
A) Standard-pitch non-ECG-gated CTPA in a 50-year-old male patient shows minor blurring of bronchovascular structures in bilateral lower lobes (score 3) and minor double-line artefacts in right lower lobe (score 3), lung image-quality artefacts are present but diagnostic (score 2). B) High-pitch PECG-gated CTPA in a 45-year-old male patient shows no blurring of bronchovascular structure with clearly discernible edges of the tracheobronchial tree, and segmental and subsegmental branches (arrows) of pulmonary arteries (score 4). Also note clear and sharp outlines of diaphragm margins, lung image quality diagnostic without any artefacts (score 4)
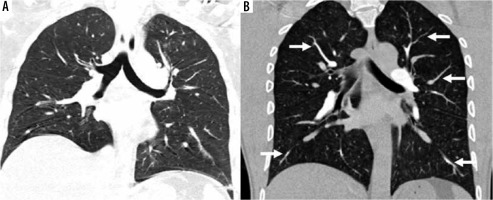
Table 3
Scores (mean with standard deviation) as measured on Likert scale for each qualitative parameter
Lung image quality
Lung image quality was better with statistically fewer artefacts in HP-PECG-gated CTPA with p-value less than 0.001 (Figure 3B). Table 4 enumerates the artefact scores as markers for quality of lung parenchyma.
Table 4
Lung image quality as measured with presence of artefacts
Quantitative parameters
Pulmonary artery enhancement
Pulmonary artery enhancement in HP-PECG-gated CTPA was comparable to SP-NECG-gated CTPA (Figure 4, Table 5). Subjectively, assessments of pulmonary vasculature until segmental and subsegmental level was better in HP-PECG-gated CTPA, but no scoring was done.
Figure 4
High-pitch PECG-gated CTPA in a 24-year-old male patient – axial and coronal images show uniform and dense opacification pulmonary arteries with quantitative enhancement in HU (MPA 396, right pulmonary artery 340, left pulmonary artery 354). Coronal image shows no intravascular shading (score 4) with visualization of subsegmental branches
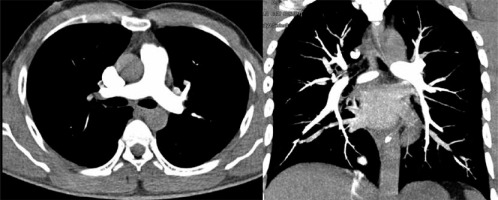
Table 5
Summary of quantitative image parameters
Other quantitative parameters
Radiation exposure in HP-PECG-gated CTPA (2.45 ± 0.80 mSv) was significantly lower (19.87%) as compared to SP-NECG-gated CTPA (3.17 ± 1.2 mSv) with p ≤ 0.007. Contrast volume was also statistically significantly lower with HP-PECG-gated CTPA (p ≤ 0.001). All the quantitative parameters with values are enumerated in Table 5.
Interobserver agreement on qualitative parameters
The interobserver agreement for assessment of various motion-related artefacts were as follows: blurring of broncho-vascular markings (κ-value 0.92), intravascular shading artefacts (0.85), and double-line artefacts (0.87). Both the readers did not differ by more than one score value in the assessment of various qualitative parameters.
Discussion
CTPA on MDCT is now an established modality for the diagnosis of patients suspected of APE [4-6]; however, diagnosis of SSPE remains challenging [7-12]. The clinical implications of diagnosis of SSPE are not known, but dia-gnosis – if possible – is desirable for proper treatment in at least a subgroup of patients with limited cardiovascular reserve [10,11,24].
Pulmonary vasculature is a contiguous structure and is anatomically divided from proximal to distal as main pulmonary artery, right and left branches, lobar, segmental, and subsegmental arteries. Segmental and subsegmental pulmonary arteries generally course parallel to segmental and subsegmental bronchi and are named according to the bronchopulmonary segments that they supply. Pulmonary artery branches move with cardiac pulsations and respiratory motion making them prone to these motion-related artefacts, compromising accuracy for diagnosis of SSPE [13-15]. Respiratory motion-related artefacts can be avoided with breath-holding or fast scanning with large area coverage, which is possible with current-generation MDCT or DSCT [16,17,25,26].
Pulmonary artery and its branches move with (a) cardiac motion that involves cyclical change in vessel diameter and length (best visualized in diastolic phase) and (b) due to transmission of pulsations by ventricle contractions through adjacent lung parenchyma [14]. These cardiac-related movements cause blurring of broncho-vascular structures, intravascular shading, and double-edge artefacts degrading image quality. After the advent of higher detectors MDCT efforts have been made to address cardiac motion-related artefacts by ECG-gated CTPA by retrospective ECG gating with success [18,19]; however, due to concerns of increased radiation exposure CTPA with retrospective ECG gating, it did not gain popularity in routine clinical practice [5,20]. With the availability of newer CT scanners, especially DSCT with faster gantry rotation time, improved temporal resolution, prospective ECG-gating CTPA with high-pitch acquisition technique was studied, and radiation exposure concerns were largely addressed [14,15,20,21,27]. Prospective ECG-gated scanning provides a single set of images in the diastolic phase of the cardiac cycle, which is the most stable with relatively little motion of cardiovascular structures [28,29]. Current CT scanners allow the selection of the range of R-R interval of ECG for triggering of the X-ray tube for data acquisition [30]. In our study we selected 60% to 80% of R-R interval for acquisition of data, and the system calculated the best phase from the preset R-R interval depending on heart rate. Prospective ECG-gated scanning, due to selective turning on of the X-ray tube by the ECG signal only in the diastolic phase, results in reduced radiation exposure as compared to retrospective ECG gating in which data acquisition is continuous throughout the cardiac cycle [29,31,32]. Usually low voltage (kVp) and high pitch acquisition leads to increased noise; however, IR reconstruction enables better quality images, compensating for parameters used to reduce radiation exposure [33-35]. In our study, patients with BMI over 40 kg/m2 were excluded and the tube voltage (kVp) was fixed at 100, but kVp value needs to be increased in case of high BMI.
In our study radiation- and contrast-optimized protocols were almost similar with difference in acquisition of data – one with high pitch and prospective ECG-gated scanning and the other with standard non-ECG gating. The results of our study reveal that HP-PECG-gated CTPA results in decreased motion related artefacts in broncho-vascular structure like blurring of broncho-vascular margins, double-line artefacts, and intravascular shading, which lead to improved (Figures 1-3) detection of SSPE with better overall lung image quality. These results are concordant with the previously reported studies with HP-PECG-gated CTPA [15,16,22].
The interobserver agreement for the assessment of qualitative parameters was statically no less (more than 0.80), and 2 readers did not differ by more than one score value in the assessment of each parameter.
In our study we used contrast-optimized protocols in both the groups, and extended contrast injection was not used for simultaneous assessment of aorta and coronary arteries as reported by Bolen et al. [15]. Pulmonary artery enhancement was statistically no less [main pulmonary artery attenuation (p = 0.92), right pulmonary artery attenuation (p = 0.50), and left pulmonary artery attenuation (p = 0.50)] even though the amount of contrast used in HP-PECG-gated CTPA was 64.75% less (45.05 ± 6.0 ml) as compared to SP-NECG-gated CTPA (74.19 ± 7.63 ml) with a p ≤ 0.001 (Figure 4). This means the pulmonary arterial vasculature was optimally opacified without compromising diagnostic quality enhancement of pulmonary vasculature. In addition, there was also better contrast opacification of segmental and sub-segmental branches in HP-PECG-gated CTPA as compared to SP-NECG-gated CTPA on subjective assessment, but no scoring was done. This enabled us to detect thrombi till subsegmental level in HP-PECG-gated CTPA 15/44 (34.09%) vis-a-vis 8/43 (18.60%) in SP-NECG-gated CTPA (Figures 1 and 2).
For radiation exposure optimization we used a low voltage setting (fixed at 100 kVp), body-adapted tube current modulation, and IR for image reconstruction for both the groups. In our study with HP-PECG-gated, CTPA showed a significant reduction (29.40%) in radiation exposure (2.45 ± 0.80 mSv) as compared to SP-NECG-gated CTPA (3.17 ± 1.20 mSv) with p ≤ 0.007, which is similar to the study conducted by Bolen et al. on 128-DSCT [15].
Our study had some limitations. The study was directed towards qualitative and quantitative assessment of image quality. The qualitative assessment was a subjective evaluation with no clear-cut or accepted guidelines present in the literature. No statistical evaluation was made regarding sensitivity and specificity in the diagnosis of SSPE. In addition, we reported an increased number of cases of SSPE in HP-PECG-gated CTPA in comparison to SP-NECG-gated CTPA. Whether this is a chance finding or statistically significant needs to be further evaluated.


