Introduction
Endovascular aneurysm repair (EVAR) is a minimally invasive procedure alternative to conventional open surgical repair in patients with abdominal aortic aneurysms. EVAR involves implanting a stent graft in the aorta to exclude the aneurysm sac from the systemic circulation and prevent rupture. Patients undergoing EVAR have a lower early and midterm perioperative mortality rate compared to patients undergoing open surgery [1,2]. However, patients after EVAR require lifelong surveillance to rapidly detect possible complications and avoid fatal consequences. The total complication rate after EVAR ranges up to 40% [3]. The most common complication is endoleak – persistent systemic blood inflow into the aneurysm sac [4].
Computed tomography angiography (CTA) stands as the reference standard for monitoring EVAR patients, yet it puts the patients at risk of receiving high doses of ionizing radiation and nephrotoxic iodine contrast [5]. To reduce the patient burden associated with CTA, both the European Society of Vascular Surgery (ESVS) and the North American Society of Vascular Surgery (SVS) recommend using color duplex ultrasound (CDUS) for postoperative surveillance [6,7]. However, some meta-analyses state that CDUS sensitivity alone is insufficient for endoleak identification in all clinical situations [8-10].
A method exhibiting outstanding sensitivity and specificity without the inherent risks associated with CTA is contrast-enhanced ultrasonography (CEUS). It employs an ultrasound contrast agent characterized by an excellent safety profile. The gas encapsulated in phospholipid bubbles improves the visualization of flow in the aneurysm sac and increases the ability to detect endoleaks [11]. However, it still entails intravascular administration and additional costs for purchasing the contrast agent.
Microvascular ultrasound (MVUS) is a novel ultrasound method that enables the visualization of slow-velocity flow without the requirement for intravenous contrast agents [12]. It applies advanced filters to reduce background artifacts and highlights microvascular blood flows [13]. Recently, various vendors have incorporated MVUS techniques into ultrasound equipment, making it readily available for clinical application. MVUS has found extensive use in diagnosing thyroid and breast lesions [14,15]. More recently, there has been an exploration of abdominal applications, including the assessment of post-EVAR patients [16].
The objective was to assess the diagnostic accuracy of microvascular flow imaging ultrasound in terms of sensitivity, specificity, negative and positive predictive value in comparison with CTA as the reference standard for the detection of endoleak after EVAR.
Material and methods
Literature search strategy
The Preferred Reporting Items for Systematic reviews and Meta-analyses of Diagnostic Test Accuracy (PRISMA-DTA) extension for DTA guidelines were used for this review [17]. We performed systematic searches of the following electronic databases: Medline (database provider PubMed), Scopus, EMBASE (database provider Ovid), and the Cochrane Library. The language was restricted to English. Two reviewers (MC and EK) independently performed searches in February 2024.
The search strategy included the following keywords: ‘ultrasound’, ‘microvascular’, ‘microflow’, ‘MVUS’, ‘SMI’, ‘MV-Flow’, ‘MFI’, ‘MVI’, ‘EVAR’, ‘endoleak’, and ‘aortic aneurysm’. The keywords contained the commercial names of MVUS techniques. All search terms were combined with Boolean operators to ensure maximal sensitivity. All papers written in English and published until January 2024 were considered potentially eligible. Articles were screened based on their titles and abstracts. All full-text copies of selected articles were systematically evaluated for eligibility using the inclusion and exclusion criteria for further study.
Inclusion and exclusion criteria
In the meta-analysis, we included all retrospective and prospective cohort and cross-sectional studies comparing MVUS and CTA for the detection of endoleaks in unselected patients after EVAR. For inclusion, all papers had to provide quantitative data sufficient to construct 2 × 2 contingency tables.
We excluded studies with less than 10 participants, studies with no consecutive enrollment of patients, studies selecting patients based on previous imaging test outcomes, and insufficient data to enable the generation of contingency tables. We also excluded all case reports, editorials, commentary, and review articles. In the case of publications from the same study population, only the latest article was included.
Data extraction
One of the review authors extracted data from the selected papers, and the second review author verified the collected data. Any discrepancies were resolved through consensus. The main outcome measures extracted from the studies were the number and type of endoleaks as well as the sensitivity, specificity, accuracy, and positive and negative predictive value (PPV, NPV) for MVUS and CTA examination at the time of follow-up. The supplementary extracted data included the author’s name, year of study, journal, study design, number of patients followed, model of aortic stents used, mean follow-up time, technical characteristics, and protocol used for MVUS and CTA scanning.
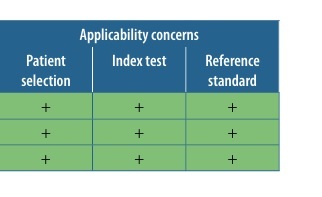
The methodological quality of the studies selected for the review was assessed using the QUADAS-2 scale [18]. The scale was utilized to examine four domains: Patient Selection, Index Test, Reference Standard, and Flow and Timing. In each domain, the risk of bias was judged as low, high, or unclear.
Statistical analysis
For each study, we prepared a 2 × 2 contingency table containing true positive, false positive, true negative, and false negative values of MVUS examination results in comparison to CTA results as a reference. Subsequently, the web application Meta-DiSc 2.0 was used to perform statistical analysis [19]. For the meta-analysis of the diagnostic accuracy of microvascular flow imaging ultrasound, a univariate random effects model was used. This model was chosen due to the small number of studies included in the review [20]. The studies were analyzed and reported by pooled sensitivity and specificity with their corresponding 95% confidence intervals (CI), diagnostic odds ratio (DOR), and heterogeneity (I2). Heterogeneity was estimated separately for sensitivity and specificity. The DOR is the odds that the test will produce correct results compared to the odds of incorrect results [21].
Results
Results of the search
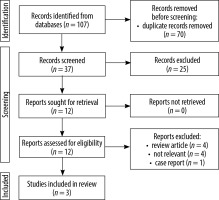
Our initial search strategy identified 107 studies. After removing duplicates, we chose 37 articles for further screening based on the title and abstract. Subsequently, we obtained the full texts of 12 articles for thorough evaluation, of which 9 studies were excluded based on inclusion and exclusion criteria. Only 3 comparative studies satisfied the selection criteria and were included in the meta-analysis [22-24]. The process of study selection is illustrated in a flow diagram (Figure 1).
Description of studies
Table 1 provides a summary of the characteristics of the included studies. The studies selected for quantitative synthesis reported a total of 209 patients with paired scans providing data comparing the MVUS with CTA. All studies were prospective in design and included consecutive patients. The Superb Micro-vascular Imaging (SMI) technology (Canon, USA) was used as an MVUS technique in all cases. The SMI examinations were conducted in two modes: the monochrome (grayscale) mode (mSMI), enhancing sensitivity by eliminating background information and concentrating on the vasculature, and the color mode (cSMI), which simultaneously displays B-mode and color information. In all studies, two or three physicians (radiologists and vascular surgeons) were responsible for the scan evaluation. In all examinations, after the MVUS examination, a CEUS examination was also performed. Injection of 1.2 to 5 ml of the second-generation ultrasound contrast agent SonoVue (Bracco, Italy) was performed. The investigation time in the CEUS modality varied between studies from 2 to 6 minutes. The CTA scanning protocol was described in studies by Cantisani et al. and Curti et al. and included triphasic scanning with the 64-layer multi-slice scanner [22,24]. The latter study did not describe the scanning protocol.
Table 1
Baseline study characteristics of included studies comparing microvascular flow imaging ultrasound (MVUS) with computed tomography angiography (CTA)
| Authors | Country | Year of publication | No. of patients | Range of follow-up | Interval between MVUS and CTA | MVUS characteristics for endoleak detection (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | PPV | NPV | ||||||
| Cantisani [22] | Italy | 2017 | 57 | 1-12 months | NR | 75% | 98% | 95% | 86% | 96% |
| Gabriel [23] | Poland | 2018 | 30 | NR | 2-3 weeks | 100% | 93.3% | 96.7% | 93.8% | 100% |
| Curti [24] | Italy | 2022 | 119 | 3-4 months | 1 month | 91.5% | 100% | 95.9% | 100% | 92.8% |
The studies compared SMI and CTA and revealed 71 and 76 endoleaks, respectively. Using SMI, type I, II, and III endoleaks were detected in 4.2% (n = 3), 91.5% (n = 65), and 4.2% (n = 3) of patients, respectively. While using CTA, type I, II, and III endoleaks were detected in 3.9% (n = 3) 83% (n = 70), and 92.1% (n = 3) of patients, respectively. Neither type IV nor type V endoleaks were reported. The sensitivity of SMI ranged 75-100%. The specificity ranged 93.3-100%. The NPV ranged 2.8-100% and the PPV ranged 86-100%. The accuracy of the method ranged 95-96.7%.
Methodologic quality of included studies
Table 2 displays the results of the methodological quality assessment for the included papers. No study showed a high risk of bias. The primary risk of bias was associated with blinding for both the index test and the reference standard. Low risk was assigned when a study explicitly mentioned blinding of observers for CTA or SMI results to the results of the other test. The risk was deemed unclear if such information was not explicitly provided by the authors. All studies employed an acceptable reference standard, and any withdrawals were explained where applicable.
Findings
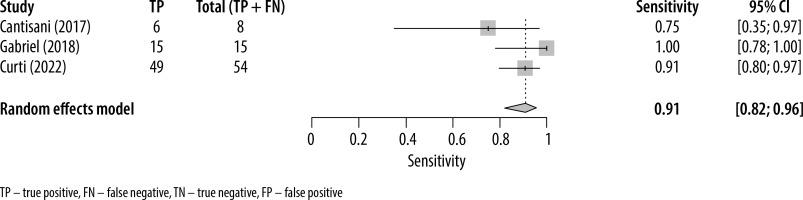
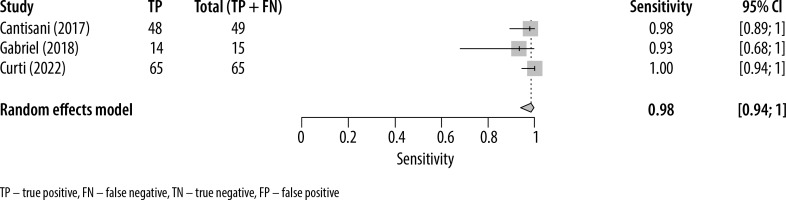
The forest plots presenting the individual study sensitivity and specificity of MVUS in detecting endoleaks are presented in Figures 2 and 3, respectively. The pooled sensitivity and specificity for the 3 studies included in the meta-analysis were 0.91 (95% CI: 0.82-0.96) and 0.98 (95% CI: 0.94-1.0), respectively. The heterogeneity (I2) of the studies with respect to sensitivity and specificity was 32% (95% CI: 15%-49%) and 9% (95% CI: –6%-23%), respectively. The pooled DOR was 635 (95% CI: 128-3140).
Discussion
The early and accurate diagnosis of endoleaks in post-EVAR patients is crucial to reduce the risk of serious complications including aneurysm-related death. Multiple imaging modalities can be employed for surveillance screening, including CTA, color duplex ultrasonography (CDUS), contrast-enhanced ultrasound (CEUS), and magnetic resonance angiography (MRA). The emerging technique that may be employed in post-operative imaging is microvascular ultrasound. This systematic review and meta-analysis were conducted to determine the pooled sensitivities, specificities, and DOR of the MVUS technique for the detection of endoleaks.
From the available literature, we were able to perform a meta-analysis of the MVUS diagnostic accuracy in detecting endoleaks after EVAR. All included studies used an MVUS solution developed by Toshiba – SMI. We found that SMI has a high sensitivity (91%) and specificity (98%) in detecting endoleaks using CTA as the reference standard.
The ESVS states that CTA remains the gold standard for patient follow-up due to its availability, uniformity of protocols, and high sensitivity and specificity for the detection of endoleaks [25]. However, some negative aspects such as the increased risk of radiation-induced cancer, nephrotoxicity related to the administration of iodine contrast agent, static view with the inability to identify the direction of blood flow, and relatively high-cost limit its frequently repeated use. A widely available alternative to CTA for post-EVAR surveillance is ultrasound. It is a non-invasive and inexpensive modality that allows repeated and reliable measurement of the maximum diameter of the aneurysm and, with the Color Doppler option, detection of flow in the aneurysm sac. However, its sensitivity was only 89% compared with CTA, and some crucial complications can be missed by CDUS [26].
The introduction of new-generation ultrasound contrast agents to the market, which are non-nephrotoxic and well tolerated by patients, has led to a significant increase in the use of CEUS in post-EVAR examinations [27]. Recent studies indicate that CEUS has an advantage over CDUS in evaluating endoleaks due to its capability to offer real-time information on the direction and velocity of blood flow [11]. Moreover, this capability enables the identification of late low-flow endoleaks, which are challenging to detect through CTA. In 2022, Karaolanis et al. [10] reported that the overall pooled rate of endoleak detection using CEUS was higher than when using CTA. However, despite its advantages, CEUS comes with certain challenges. It requires an experienced operator, administering intravenous substances, and the use of an expensive contrast agent, which in total increases the cost of the procedure.
In 2014, the first MVUS software algorithm, called Superb Micro-vascular Imaging, was introduced by Toshiba [13]. SMI offers several benefits over conventional CDUS such as low-velocity flow visualization, high resolution of the image, minimal motion artifact, and high frame rates. The generated images are similar to those obtained with intravenous contrast injection. To date, only three studies have applied this technique in the surveillance of patients after EVAR. In a group of eight patients with type II endoleaks, Cantisani et al. observed that SMI demonstrated lower sensitivity compared to CEUS and CTA (75%). However, SMI was deemed reliable for the classification of endoleaks [22]. Gabriel et al. analyzed a larger patient group with various types of endoleaks (n = 15) using SMI, CEUS, and CTA. They reported comparable results for endoleak evaluation, including similar sensitivity, specificity, and accuracy values among the three methods [23]. In Curti et al.’s study, there were 54 detected type II endoleaks. When compared with CTA, SMI exhibited high sensitivity (91.5%) and specificity (100%) for identifying endoleaks. In only five cases, SMI failed to detect endoleaks that were visible on CTA [24]. These findings suggest that SMI can be a suitable method for endoleak detection after EVAR, especially in patients with contradictions to CEUS (acute coronary syndrome or unstable angina) or CTA (renal failure).
The limitations of the SMI technique align with those of CEUS and CDUS, being impeded by factors such as intestinal gas, high body mass index, and potential subcutaneous emphysema. Other drawbacks encompass operator dependency, and an inability to assess sealing zone length, stent graft overlap, and device migration. Also, in comparison to CEUS, SMI offers only qualitative assessment, detecting the presence of an endoleak without indicating flow direction.
Strengths and weaknesses of the review
Our review adhered to the standards established in the Preferred Reporting Items for Systematic reviews and Meta-analyses of diagnostic test accuracy. A thorough literature search was conducted with clinically relevant inclusion criteria. Despite the small number of included papers, all studies were prospective, similar in design, and used the same technology – SMI developed by a single manufacturer, ensuring low heterogeneity. We used the QUADAS-2 tool to evaluate the methodological quality of the included studies, revealing acceptable quality in most of them.
The results should be interpreted in the context of the review’s limitations. The meta-analysis included only 3 studies with 209 paired scans. Due to the inclusion of only a few eligible studies, a univariate meta-analysis was performed instead of a bivariate analysis. Univariate random-effect models were employed, pooling sensitivity and specificity separately and disregarding potential correlations between the two measures [28]. In two studies potential observer bias might have accounted for the results as it was unclear whether the ultrasound operator performing SMI was blinded to CTA results and CEUS examination.
Conclusions
In conclusion, our findings underscore the potential of SMI in detecting endoleaks after EVAR, revealing high diagnostic accuracy and appearing to be a promising screening test. It addresses some of the shortcomings of conventional CDUS, particularly in terms of sensitivity, and eliminates the need for intravenous contrast administration. While our results are encouraging, the available data are sparse, and further research is warranted to explore the broader application of SMI and other vendors’ MVUS, providing a more comprehensive understanding and establishing robust criteria for its role in clinical practice.