Introduction
The establishment and maintenance of effective vascular access play a vital role in ensuring the long-term success of dialysis therapy. Haemodialysis provides various options for vascular access, including native arteriovenous fistulas (AVFs), arteriovenous grafts (AVGs), and central intravenous catheters. However, the use of catheters should be avoided due to their association with greater risks of mortality, infection, cardiovascular events, and hospitalisation compared to AVFs or AVGs [1,2].
Arteriovenous fistulas have garnered strong endorsement as the favoured vascular access choice for extended haemodialysis, with support from reputable organisations such as the National Kidney Foundation (NKF), the Euro-pean Best Practice Guidelines Expert Group (EBPG) on Haemodialysis, and the Society for Vascular Surgery (SVS) [3-5]. Functioning AVFs exhibit a lower incidence of mortality, infection, and hospitalisation compared to AVGs [1,6]. Furthermore, AVFs require fewer interventions to keep their patency over the long term [7].
Arteriovenous fistula and AVG dysfunctions emerge as the primary factors leading to hospitalisation among haemodialysis patients [6]. AVF dysfunction may manifest through various issues like stenosis, thrombosis, aneurysm formation, pseudoaneurysms, infections, arterial steal syndrome, or the development of seromas/haematomas. It is crucial to promptly identify the underlying cause to ensure uninterrupted dialysis and address fistula dysfunction [4].
The use of colour and spectral Doppler ultrasound (CDUS) examinations is useful in assessing AVF maturation and identifying potential causes of dysfunction or presenting symptoms. An in-depth understanding of the patient’s operative history and anatomy, notably any aberrant anatomical features, enhances the effectiveness of ultrasound evaluations [8,9]. However, it is worth noting that the diagnostic utility of CDUS is limited in cases of central venous issues due to the existence of the clavicle and sternum, as well as its dependence on the operator’s skills [10].
Presently, digital subtraction angiography (DSA) is considered the premier benchmark for evaluating fistula patency and associated complications. DSA, which is not more aggressive than a syringe piercing for dialysis, offers the advantage of being combined with endovascular interventions to address stenoses. However, it is crucial to acknowledge that DSA exposes patients to ionising radiation and iodinated contrast agents [11].
While numerous previous studies have explored the diagnostic accuracy of Doppler ultrasound in assessing haemodialysis fistulas, none have delved into the reproducibility of Doppler ultrasound results among experienced radiologists or examined the specific diagnostic challenges encountered in AVF/AVG complications. Without stronger evidence of the reproducibility of Doppler results, their clinical utility may become limited. Consequently, this multicentre prospective study, involving multiple independent radiologists, was conducted to evaluate the interobserver agreement and the diagnostic performance of Doppler ultrasound, using digital subtraction angiography as the standard reference.
Our hypothesis asserts that radiologist agreement concerning stenotic and steal syndrome complications in haemodialysis fistulas is a critical factor influencing the diagnostic effectiveness of Doppler ultrasound.
Material and methods
Ethical statement
This prospective multicentre cross-sectional study received approval from the Institutional Review Board (approval number: ZU-IRB# 11214). Each patient provided written informed consent, and all procedures adhered to the principles outlined in the Declaration of Helsinki.
Patient population
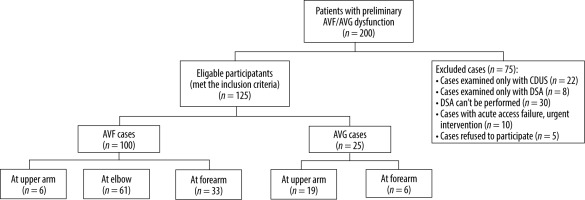
This study was conducted during the period from May 2022 to January 2023. A total of 200 patients initially dia-gnosed with AVF/AVG dysfunction were referred to the radiology department across 3 different institutions.
The inclusion criteria for this study involved patients who encountered repeated difficulties with access cannulation, frequently experienced prolonged bleeding following cannulation, reported a decreased shunt thrill sensation, or exhibited symptoms suggestive of aneurysm, thrombosis, occlusion, or severe hand numbness during dialysis. Conversely, the exclusion criteria comprised cases that had been solely assessed using CDUS, those exclusively evaluated with DSA, situations where DSA was not feasible, instances requiring immediate intervention due to acute access failure, and cases in which patients refused participation (Figure 1).
These criteria resulted in a final cohort of 125 participants, comprising 71 men and 54 women, with a mean age of 55 years.
All study participants underwent a comprehensive evaluation process, including a detailed medical history review, thorough general and local examinations, CDUS assessments, and ultimately DSA as the definitive diagnostic procedure.
The time interval between the initial CDUS examination and the subsequent DSA assessment had a mean duration of 10 days, ranging 3-18 days.
CDUS examination and image analysis
Colour Doppler ultrasound workups were conducted using a Doppler ultrasound scanner (Philips, EPIQ 7G) equipped with a 5-10 MHz linear probe. Three highly experienced radiologists, each possessing over 15 years of ultrasound and Doppler experience, independently carried out all Doppler US examinations. They were blinded to the clinical data of the patients. The operator assumed a position in front of the patient or on the access side, while the patient was seated or in a semi-upright posture, a positioning that optimises gravity’s assistance in vein dilation.
The comprehensive examination included the meticulous evaluation of the inflow artery, the site of fistulous connection, and the outflow veins, extending as regards the subclavian vein, along with the remaining arterial vasculature caudal to the AVF/AVG. All vessels underwent a thorough examination in both transverse and longitudinal planes, utilising greyscale and colour images. Initially, B-mode imaging was employed to settle the location and variety of the fistula, identify wall echogenicity and dilatations, and measure vessel diameters. Subsequently, colour Doppler images were acquired to evaluate blood flow direction. Eventually, spectral Doppler assessment was performed longitudinally, with the wall filter set at 50-100 Hz and the sample size kept below 5 mm, positioned at the centre of each vessel. Except for flow volume measurement, the sample volume had to encompass the entire vessel diameter, ensuring the inclusion of low-velocity layers adjacent to the vessel wall. The spectral waveform was adjusted for angle correction, ensuring that Doppler angles of insonation remained below 60°, and spectral waveforms were recorded at every station. Angle correction is considered accurate for insonation angles equal to or larger than 60° for improved diagnostic performance; nonetheless, errors of up to 20-30% may be evident in recorded velocities [12,13].
Numerous variables were traced at the afferent artery and AV anastomosis site, including arterial dia-meter 2 cm proximal to the fistula site, fistula type, diameter, subcutaneous depth, flow volume, peak systolic velocity (PSV), and end-diastolic velocity (EDV). Furthermore, scanning of the proximal, mid, and distal draining veins was conducted, encompassing measurements of diameters, patency, and mean velocities. PSV and EDV were quantified in grafts from the venous and arterial junction levels and within it. In cases of identified stenosis, the degree of stenosis was calculated. Waveforms and PSVs were documented wherever velocity increase or turbulence was observed. Stenosis was settled when there was a diminution in vessel diameter of more than 50% and an increase in the PSV ratio (PSV in the stenotic area/PSV upstream of the stenotic area) exceeding 2 : 1 in the afferent artery/draining vein or greater than 3 : 1 in the anastomotic area. Throughout our analysis, we repeatedly modified the pulse repetition frequency (PRF) to counteract the increased velocities noted in the stenotic portions and get rid of the aliasing. Stenoses were graded as juxta-anastomotic (stenosis within 5 cm of the anastomosis), outflow vein, central venous stenosis, or inflow artery stenosis (Figures 2-4, Supplementary Figures S1, S2).
Figure 2
A 55-year-old female patient with a native brachiocephalic haemodialysis fistula who complained of difficulties during cannulation. A, B, C) Colour Doppler ultrasonography (CDUS) images depict a short juxta-anastomotic segment of the cephalic vein, showing moderate stenosis (calibre = 2.5 mm) with colour Doppler aliasing, an increased peak systolic velocity (PSV) to 410 cm/s and proximal venous aneurysmal dilation (calibre = 18 mm). D) Digital subtraction angiography (DSA) confirms the presence of a correlated narrowed and opacified segment along with aneurysmal dilatation in the remaining course of the cephalic vein
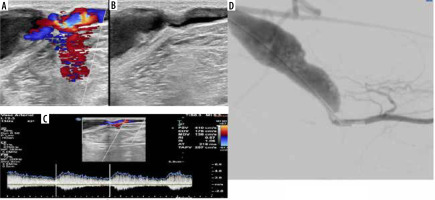
Figure 3
A 67-year-old male patient with a brachiocephalic native haemodialysis fistula presented with clinically decreased shunt thrill. A) Colour Doppler ultrasonography (CDUS) demonstrates a notable change in the calibre of the cephalic vein just proximal to its confluence with the axillary vein, characterized by attenuated calibre and weak flow. B) Digital subtraction angiography (DSA) corresponds to CDUS findings, showing a significant narrowing in the proximal segment of the cephalic vein. C) Restoration of adequate flow following therapeutic DSA balloon dilatation
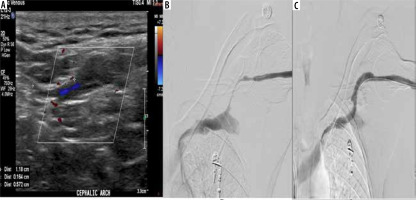
Figure 4
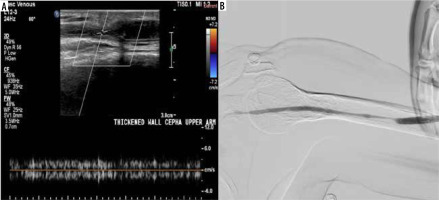
A 72-year-old female patient with a brachiocephalic native haemodialysis fistula reported difficulties during cannulation. A) Colour Doppler ultrasonography (CDUS) exhibited a marked alteration in the cephalic vein calibre in the upper arm, accompanied by a reduction in peak systolic velocity (PSV) and the loss of arterialisation in the venous waveform. B) Digital subtraction angiography (DSA) uncovered a relatively long segment of reduced cephalic vein calibre in the upper arm, in agreement with the ultrasound findings
For distal arteries, examinations were performed to identify arterial steal, with flow direction recorded both before and after gentle compression of the graft to detect any reversal of flow direction. Other pathologies, including thrombus (Figures 5, Supplementary Figures S3), aneurysm, pseudoaneurysm, and perivascular complications such as oedema, seroma, and haemorrhage, were also meticulously documented.
Figure 5
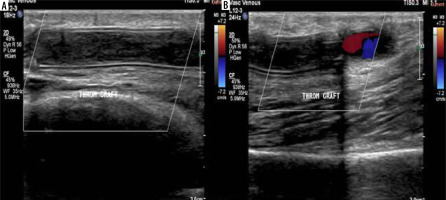
A 50-year-old female patient with a brachio-axillary haemodialysis synthetic graft experienced difficulties during cannulation. A, B) Colour Doppler ultrasonography (CDUS) revealed a synthetic graft with double echogenic walls in situ, and no colour flow was detected throughout its length. However, colour flow was observed at the junction point of the graft with the native vessel, indicating graft thrombosis
Data collected from the 3 participating institutions were centrally compiled for subsequent analysis. Inter-reviewer agreement (IRA) assessments were conducted among 3 independent reviewers to evaluate the diagnostic features of AVF/AVG complications. To estimate the diagnostic efficacy of CDUS in forecasting the primary cause of non-functioning AVF/AVG, evaluations by 3 independent reviewers were amalgamated to arrive at a final diagnosis. Any disparities among reviewers were resolved through consensus during this process.
Reference standard
Digital subtraction angiography was conducted by a skilled interventional radiologist, namely M.N., using a specialised Siemens Artis Q.zen Angio suite. Importantly,the radiologist did not have entree in the results of the formerly conducted CDUS examinations.
The procedure involved backward puncturing of the venous segment of the access, followed by the insertion of a 4F dilator. To capture comprehensive images of the entire venous outflow, manual injections of 5-10 ml of nonionic contrast material (Ultravist, Iopromide, 300 mg I/ml)were repeatedly administered. Images of both the access and its distal arterial inflow were acquired using either manual compression or flow interruption via a cuff on the venous outflow. In cases where this approach failed to provide adequate visualisation of the access or feeding access, the 4F dilator was replaced with a 4F straight catheter, and its tip was inserted caudally into the inflow access. To enhance visualisation, zooming series and oblique projections of possible stenosis were attained. If multiple cannulation procedures were required to accurately depict the access and its feeding and draining pathways, the interventional radiologist recorded the trials of penetrations and the triggers for the recurrent cannulation.
Statistical analysis
MedCalc (version 11.1; MedCalc, Mariakerke, Belgium) was used to collect the data. The Mann-Whitney U test was utilised to compare categorical variables, and the Fleiss and weighted kappa (κ) tests were employed to evaluate the inter-observer agreement. Digital subtraction angiography (DSA) served as the reference standard for this analysis. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of CDUS were computed to assess its efficacy in detecting various vascular segments, including significant stenosis, thrombosis, aneurysms, pseudoaneurysms, distal arterial steal, and perivascular complications. Notably, significant stenosis was further categorised into subgroups based on their specific locations, and the same set of statistical parameters (sensitivity, specificity, PPV, NPV, and accuracy) was determined for each of these subgroups.
To evaluate inter-reviewer agreement (IRA), Fleiss kappa (κ) statistics were employed, taking into account the input from multiple reviewers. The interpretation of κ values followed this scale: 0.01-0.20 denoted poor agreement, 0.21-0.40 indicated fair agreement, 0.41-0.60 represented moderate agreement, 0.61-0.80 signified good agreement, and 0.81-1.0 indicated very good agreement.
Results
Our study included 125 patients experiencing malfunctioning AVF/AVG access. Among these cases, 25 (20%) were diagnosed with AVG dysfunction, while the remaining 100 (80%) had AVF dysfunction. Of the 19 cases involving AVGs, 6 comprised brachiobasilic loop and brachioaxillary straight grafts at the arm level, and 6 were radiobasilic loop grafts at the forearm level. Among the 100 native AVFs, 61 were brachiocephalic AVFs, 6 were brachiobasilic AVFs, and 33 were radiocephalic AVFs. A summary of the distribution of cases by gender and age can be found in (Table 1).
Table 1
Age and sex distribution among the studied group
| Value (N = 125) | Parameter | |
|---|---|---|
| 65 (52%) | Female | Sex, n (%) |
| 60 (48%) | Male | |
| 43.74 ± 8.58 | Mean ± SD | Age (years) |
| 26-58 | Range | |
When considering the distribution of presenting symptoms that raised suspicion of dialysis access dysfunction, the most frequent symptom was repeated problematic cannulation of the access, observed in 81 patients, constituting 65% of the study sample. This was followed by frequent prolonged bleeding after cannulation and decreased shunt thrill, each accounting for 16% of the cases. The least common presenting symptom was extreme hand pain during dialysis, affecting 3% of the study sample (Table 2).
Table 2
Presenting symptoms of the studied patient population
| Percentage | Number | Presenting symptoms |
|---|---|---|
| 65 | 81 | Problematic cannulation of the access |
| 16 | 20 | Frequent prolonged bleeding after cannulation |
| 16 | 20 | Decreased shunt thrill |
| 3 | 4 | Extreme hand pain during dialysis |
Inter-observer agreement regarding complications of AVF/AVG was very good for the identification of thrombus (κ = 1.0), seroma (κ = 0.953), aneurysm (κ = 0.851), and pseudoaneurysm (κ = 0.851). It was considered good for the detection of juxta-anastomosis stenosis (κ = 0.751) and feeding artery stenosis (κ = 0.638). However, the agreement was fair for identifying draining vein stenosis (κ = 0.380) and distal arterial steal syndrome (κ = 0.210) (Table 3).
Table 3
Inter-reviewer agreement for fistula complications
The overall diagnostic performance of CDUS exhibited 86% sensitivity in identifying stenosis, with a spe-cificity of 99.1%, a PPV of 96.5%, a NPV of 97%, and an accuracy of 94.3% (Table 4).
Discussion
End-stage renal failure represents a significant medical, societal, and economic challenge. Patients in this condition require dialysis treatments to eliminate toxins from their vascular system, achieved by placing 2 needles in a vein, typically in the upper extremity [14]. The long-term survival of individuals on haemodialysis is contingent upon the efficacy of dialysis achieved through well-placed vascular access. Despite AVFs being the ideal choice for vascular access, clinical practice continues to grapple with a significant percentage of maturation malfunction [15].
This study is unique because it explores the inter-reviewer agreement (IRA) among experienced radiologists regarding CDUS results, a topic not previously investigated. Our findings illuminated exceptional IRA in identifying thrombus, seroma (κ = 1.0), aneurysm (κ = 0.953), and pseudoaneurysm (κ = 0.851). It showed good agreement in recognizing juxta-anastomosis stenosis (κ = 0.751) and feeding artery stenosis (κ = 0.638). However, the IRA displayed only fair agreement when detecting draining vein stenosis (κ = 0.380) and distal arterial steal syndrome (κ = 0.210). These results provide additional support for the clinical utility of CDUS.
In comparison to previous studies, our results aligned with those of Cansu et al. [16], who reported CDUS sensitivity of 74.2% in detecting stenosis, along with a specificity of 90.6%, a PPV of 89.6%, a NPV of 76.3%, and an overall accuracy of 82.6%. Additionally, Doelman et al. [17] found CDUS sensitivity of 91% for stenosis detection, with a specificity of 97%, PPV of 91%, and NPV of 97%. While we did not assess the diagnostic accuracy of CDUS for different stenosis locations, Chandra et al. [18] emphasised CDUS’s accuracy in pinpointing fistula stenosis location, particularly when placed in the inflow access, fistula itself, or outflow access, underscoring its role in intervention planning.
Notably, our study reported 100% sensitivity for diagnosing thrombosis, aneurysms, and pseudoaneurysms, aligning with previous research [16-20]. However, our reported sensitivity for identifying distal arterial steal syndrome was 66.7%, with a specificity of 67%, PPV and NPV of 66.6%, and an accuracy of 66.7%.
Conclusions
Our study concurs with Abdelaziz et al. [19] in highlighting CDUS as a noninvasive diagnostic approach for the prompt picking of AVF complications. It serves as a suitable first-line imaging modality for nonfunctional AVF due to its cost-effectiveness and accessibility. Additionally, we provide evidence of reproducibility, encouraging the diligent use of CDUS in AVF and AVG evaluation for early complication detection and management guidance.
While this study boasts a large, prospective, multicentre design that minimises selection bias inherent in retrospective studies, it does have limitations. Notably, all CDUS interpretations were conducted by highly skilled radiologists, clearly affecting diagnostic accuracy and explaining the substantial inter-rater reliability. Consequently, further research is warranted to evaluate the accomplishment of this classification when implemented by radiologists with less training.