Introduction
Middle cerebral artery (MCA) aneurysms account for 18-22% of all intracranial aneurysms and are the third most common location for aneurysmal subarachnoid haemorrhage [1,2]. In the case of small unruptured MCA aneurysms, the indication for treatment should be made carefully because the risk of rupture of these lesions is very low [3]. There are, however, some clinical situations, e.g. an enlarging aneurysm on repeat imaging, when early treatment is recommended [4]. Before an interventional management is considered, the natural history of small unruptured aneurysms must be compared with potential procedure-related complications. Traditionally, in regard to MCA aneurysms, microsurgical clipping has often been the first-line treatment method [5-7]. Although the endovascular coiling of MCA aneurysms is controversial, there is increasing interest in treating these lesions via this approach [6-14].
In the literature there are a limited number of studies concerning endovascular coiling of small unruptured aneurysms [15-17]. In fact, there seems to be no published study entirely dedicated to endovascular embolisation of small unruptured MCA aneurysms. The purpose of the current study is to report our experience with the endovascular coiling of small (< 5 mm) unruptured MCA aneurysms, with special regard to immediate and long-term radiographic and clinical outcomes and procedure-related complications.
Material and methods
Patient population
Between February 2008 and March 2015, 19 patients harbouring 20 small (< 5 mm), unruptured MCA aneurysms were treated at our centre via endovascular approach. There were 15 (78.9%) females and four (21%) males. The patients’ ages ranged from 47 to 73 years (mean 57.6, SD 6.6). Seven patients underwent standard coil embolisation, and 12 patients underwent stent-assisted coiling. All the patients included in the present study were treated for the first time via endovascular approach. There were no retreatment procedures. For this retrospective study Ethics Committee approval and informed patient consent were waived.
Aneurysm characteristics
The aneurysm size, defined as the maximal height of the dome, ranged from 1.9 mm to 4.7 mm (mean 3.8, SD 0.7). Patient selection for treatment with stents was based only on the angioarchitectural characteristics of each aneurysm. Thus, only the wide-neck aneurysms, defined as those with aneurysm neck diameter ≥ 4 mm and/or those with dome-to-neck ratio < 1.5, were considered for this treatment.
Endovascular techniques
Because all the aneurysms were depicted by computed tomography angiography at admission, both conventional and rotational digital subtraction angiographies (DSA) were performed in every case to evaluate accurately the aneurysmal configuration, neck size, and the width and height of the aneurysm. All the procedures were performed by experienced interventional radiologists, and always after neurosurgical consultation. The patients were treated under general anaesthesia. Anti-platelet premedication, consisting of a 75 mg loading dose of acetylsalicylic acid and an additional 75 mg clopidogrel in the case of planned stent-assisted coiling, was performed every day for five days before coiling.
Following selective catheterisation of the aneurysm lumen, another microcatheter was placed distally in the M2 segment for stent deployment. A neuroform stent (Stryker) was advanced over the microguidewire and deployed when the precisely targeted location was confirmed. Then, the aneurysm was coiled using Stryker Detachable Coils in the same session with the help of the previously deployed microcatheter. The procedures were usually performed in a bi-plane angiography suite. Once it was technically feasible, an attempt was made to achieve complete occlusion in the first treatment session. During the procedures heparinised saline was continuously infused into the arterial line. After embolisation, the patient was transferred to the intensive care unit for clinical observation and monitoring of medical parameters. At that time, systemic heparin 15,000 IU for the next 24 hours was administered intravenously to raise the activated partial thromboplastin time 2-3 times above normal values. Patients after uncomplicated treatment were typically discharged from hospital on postoperative day 3. Antiplatelet therapy consisting of 75 mg acetylsalicylic acid was applied postoperatively every day with the intention of administering for the rest of the patient’s life. In the case of stent-assisted coiling an additional 75 mg of clopidogrel was applied postoperatively every day for six weeks.
Angiographic and clinical analysis
A control angiography was obtained immediately after the embolisation and at a minimum follow-up of six months. Anatomical results were evaluated using three-class classification according to Raymond et al. (i.e. complete obliteration, residual neck, and residual aneurysm) [18]. A lack of contrast filling within the entire aneurysm and the residual filling in the aneurysm neck were categorised as complete and near-complete occlusion, respectively, whereas incomplete occlusion was defined as any opacification of the aneurysm sac. In cases of complete aneurysm obliteration at control angiogram performed a minimum of six months after coiling, the patient was not followed-up any longer, except for selected cases (e.g. endovascular treatment of accompanying cerebral aneurysms). Recanalisation was defined as any increase in aneurysmal filling at follow-up. Once there was enough space for placement of an additional coil, retreatment was considered.
Regarding clinical evaluation, modified Rankin Scale (mRS) scores were recorded at discharge from hospital and during the last angiographic follow-up period. Patients who did not undergo control angiography were interviewed by telephone if it was possible.
Detailed patient characteristics as well as outcomes are presented in Table 1.
Table 1
Patient and aneurysm characteristics, occlusion rates, and outcome for patients with small unruptured middle cerebral artery aneurysms treated by coiling
Results
Angiographic results and retreatment
Instant post-embolisation angiogram revealed a complete aneurysm occlusion in 18 (90%) cases. A near-complete aneurysm occlusion was performed in one case (5%), and incomplete occlusion in another one (5%). Primary inadequate occlusion of aneurysms resulted from intraprocedural aneurysm rupture in one case. In another case, of near-complete occlusion, it was impossible to occlude the aneurysm with the shortest available coil.
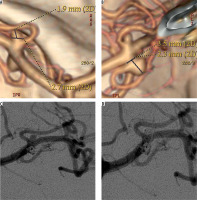
Angiographic follow-up was achieved in 17 (89.4%) cases and ranged from six to 78 months, with a mean of 19 months. In 16 (94.1%) cases no change in the degree of occlusion at initial angiographic follow-up was observed. An illustrative example is presented in Figure 1. The recanalisation rate was 5.9% (1/17). There were no retreatment procedures.
Figure 1
53-year-old female patient presented with two small unruptured middle cerebral artery (MCA) aneurysms. A, B) Computed tomography angiograms showed two small (< 5 mm) MCA aneurysms. C, D) Control digital subtraction angiography following endovascular coiling, showed the complete occlusion of the two aneurysms. Follow-up angiogram (not shown) performed after 78 months confirmed the complete obliteration of the lesions
Clinical results and complications
The procedure-related mortality rate of 5% (1/20 procedures) was associated exclusively with an event of intraprocedural bleeding of the aneurysm. Perforation of the aneurysm occurred during insertion of the first coil. The embolisation was not continued. Urgently performed CT scan revealed massive subarachnoid haemorrhage with intraventricular haemorrhage. The patient expired as a consequence of delayed cerebral ischaemia associated with SAH. There was a case of a coil prolapse into the parent artery without any adverse neurological consequences.
Clinical follow-up was achieved in 17 (89.4%) cases and performed at 6-78 months (mean 24.1 months) after coiling. Analysis showed no change in clinical status in all followed patients.
Discussion
The traditional treatment for MCA aneurysms has been microsurgical clipping, because these aneurysms can be easily approached surgically and manipulated after splitting the Sylvian fissure [19]. Surgical clip ligation in the case of MCA aneurysms results in effective and durable aneurysm repair with the rates of complete occlusion above 90% and good neurological outcomes, and this form of treatment has been commonly preferred to endovascular coiling [19,20]. In the literature, however, there is no randomised, controlled trial that has compared the efficacy and safety of MCA aneurysm treatment between the two above-mentioned modalities [7,19-21]. Even on the basis of a multicentre, randomised ISAT trial involving ruptured intracranial aneurysms, it is impractical to establish the optimal method of management of MCA aneurysms because these lesions were underrepresented in this study [19,21,22]. The two reported meta-analyses on unruptured MCA aneurysms performed by Smith et al. and Blackburn et al. showed that the surgical clipping is associated with higher occlusion rates compared to coil embolisation [20,23]. According to the study by Smith et al., the microsurgical procedures yielded slightly lower unfavourable clinical outcomes, but in the report of Blackburn et al. the functional outcomes did not differ significantly between the two groups [20].
Although the coil embolisation of MCA aneurysms is controversial, there is increasing interest in treating these lesions via endovascular approach [6-11]. Advances in endovascular techniques such as rotational angiography with three-dimensional reconstruction allows improved visualisation of MCA aneurysms and arterial branches before endovascular treatment [7,20,24]. Development of remodelling techniques such as balloons and stents, as well as improvement of preventive protocols of anticoagulation, have resulted in increased application of endovascular therapy of MCA aneurysms [7,20,24]. It is reported that in properly selected patients, endovascular coiling of MCA aneurysms should be considered, and satisfactory aneurysm occlusion is achieved in 62.5% to 96.8% of patients, with a complication rate from 3.8% to 20% [7,8,25,26].
One of the fundamental limitations of endovascular coiling of MCA aneurysms is the frequently complex anatomy of these lesions [7,19]. These aneurysms often have wide necks, frequently incorporate one or both M2 branch vessels, and may have branches originating from the base or side wall of the aneurysm [7,19]. Coil embolisation of MCA aneurysms with unfavourable morphology may be associated with increased risk of branch occlusion [7,19,20,24].
In the case of small unruptured aneurysms the indication for intervention therapy is still under debate. The natural history of these lesions has not yet been conclusively investigated, and there is still uncertainty if coil embolisation of small unruptured aneurysms significantly reduces the incidence of haemorrhage [15-17]. According to the prospective ISUIA study published in 2003 the five-year cumulative rupture rate of MCA aneurysms < 7 mm is 0% [3]. Other studies on the natural history of unruptured aneurysms revealed a somewhat higher rupture risk in comparison to the 2003 ISUIA trial in the case of MCA aneurysms, but the risk is still very low [27,28]. The definite cut-off size above which the cerebral aneurysms should be unequivocally recommended for treatment has not yet been determined [15]. Nonetheless, selected patients with asymptomatic small aneurysms should be considered for treatment. These include patients with aneurysm growth, younger age, previous subarachnoid haemorrhage from another aneurysm, family history of aneurysm in first-degree relatives, certain morphological characteristics of aneurysm, or psychological factors [4,29]. When deciding how to treat patients with small unruptured aneurysms, surgical clipping as well as embolisation procedures may be taken into consideration. In the literature there are no studies directly comparing microsurgical clip ligation and endovascular embolisation of small asymptomatic unruptured aneurysms [16]. In view of occlusion durability, surgical clipping is considered to be applied in younger patients with a long life expectancy [29]. In older patients coil embolisation might be preferable; however, it is doubtful if the risk of treatment in such patients outweighs the rupture risk for small unruptured aneurysms [29]. Nonetheless, coil embolisation of small unruptured aneurysms may be performed with an acceptable occlusion rate and low degree of recurrence [29]. According to previous reports the initial complete or near-complete occlusion rate of small unruptured aneurysms treated via endovascular approach is 94.7-98.6% [15-17]. In comparison to others, small MCA aneurysms are associated with lower feasibility of endovascular coiling [16]. Oishi et al. reported that the risk of technical failure during endovascular coiling of small (< 10 mm), unruptured cerebral aneurysms was highest in MCA aneurysms, with a failure rate of 11.1% [16]. The results of our study with the 95% rate of the initial complete or near-complete aneurysm occlusion showed that small aneurysms on MCA may be coiled with satisfactory angiographic outcome.
The previously reported recanalisation rate during endovascular embolisation of small unruptured aneurysms ranges between 2.9% and 9% [17,30,31]. Our results, with a recanalisation rate of 5.9%, are comparable with these studies. It should be noted that two cases of complete aneurysm obliteration from our database with angiographic follow-up of 48 and 78 months are proof that coiling of small unruptured MCA aneurysms may have long-term durability. To our knowledge, the present two cases of coiled small unruptured MCA aneurysms harbour the longest angiographic follow-up compared with the literature to date.
It is obvious that in the case of small unruptured aneurysms the risk of treatment-associated complications should be lower than the rupture risk of the aneurysms [29]. Im et al. presented a 10.1% risk of complications during treatment of small unruptured aneurysms, with 0.27% morbidity and 0% mortality rate [15]. In their report of 500 small unruptured intracranial aneurysms, Oishi et al. experienced 38 incidents of complications (7.6%) with morbidity and mortality rates of 0.8% and 0.2%, respectively [16]. The 5% risk of procedure-related mortality presented in the current study seems to be quite high, especially considering the low rupture rate of small MCA aneurysms. This relatively high complication rate should be taken into consideration when choosing this form of treatment for small unruptured MCA aneurysms. Although it is not uniformly established, the coiling of small aneurysms in comparison to larger lesions may be associated with higher risk of intraprocedural aneurysm rupture [17,32,33]. It may result from the more restricted space available for movement of a microcatheter within the small aneurysm, which results in lesser stabilisation of a microcatheter position [17,32,33]. This complication may have benign clinical course but also may be associated with fatal clinical consequences, as was presented in the case of aneurysm perforation from the current study [17]. In our case the perforation was performed with the first coil deployed into the aneurysm lumen, and it was impossible to stop the bleeding with prompt complete embolisation. This resulted in massive intracranial bleeding with subsequent death of the patient. There is a theory that a higher rupture rate during coiling of small aneurysms may be associated with a higher stiffness of the initial segment of a coil, i.e. the segment between the tip of the microcatheter and that of the coil that contacts the aneurysm wall [32]. The so-called bending stiffness of a coil is inversely proportional to the length of the coil segment, and the stiffness of the initial coil segment is supposed to be higher in smaller aneurysms in comparison to larger ones. Therefore, the risk of aneurysm perforation with a coil may be higher in smaller aneurysms [32].
Another adverse event that may take place during aneurysm coiling is coil protrusion into the parent artery. Coil prolapse may be associated with thromboembolic events, and the risk is high, but a stent deployment into the parent artery or coil retrieval is generally recommended for cases of severe protrusions [34]. In the literature, thromboembolism has been the most frequent complication accompanying MCA aneurysm embolisation, and it may have serious clinical sequelae because the MCA branches often supply eloquent branches [6,7]. In the case of a clinically silent coil prolapse presented in the case of our database no interventional treatment was initiated. Because the blood flow in the parent artery was maintained we decided to treat the patient conservatively with aggressive antiplatelet therapy, as was presented in previous reports [35,36]. The clinical and radiographic course of our patient with 20-month follow-up proceeded uneventfully, suggesting that the expectant management is likely to be a safe treatment option for non-massive coil protrusions in small MCA aneurysms.
A limitation of the current study is its retrospective and observational character. There was also a patient-selection bias, because all the cases reported in this series were from a single institution.
Conclusions
Endovascular treatment of small unruptured MCA aneurysms is feasible with satisfactory angiographic outcome and low recurrence rate. However, procedure-related complications resulting in severe clinical consequences are not negligible and should be taken into consideration, especially in terms of benign natural course of these lesions.


