Introduction
Hirayama disease (HD) is a form of non-progressive asymmetrical amyotrophy of the upper limb muscles of the hand and forearm, which are innervated by the C7, C8, and T1 nerve roots [1]. It mainly affects the young population (15-25 years) with a predilection for males. The amyotrophy is unilateral in most patients, asymmetrically bilateral in some, and rarely symmetrical. The cause of myelopathy is the anterior displacement of the posterior cervical dural sac during flexion of the neck, which leads to the compression of the cervical spinal cord. The pathophysio-logy behind this is still not clear; however, it is believed that there is insufficient growth of the cervical dura mater relative to the cervical spinal cord during adolescence. Thus, it becomes displaced forward during neck flexion leading to cord compression, ischaemia, and subsequent necrosis of the anterior horn cells of the spinal cord at the C7-T1 level. Ischaemia and necrosis is due to the microcirculatory changes that take place in the branches of the anterior spinal artery as a result of repeated episodes of flexion of the neck [1-6]. The increased range of motion (ROM) of the cervical spine in these patients during fle-xion as compared to the normal population is also hypo-thesized to play a role in the progression and severity of HD [7,8].
The asymmetric amyotrophy of the upper limb muscles can also be seen in other conditions like amyotrophic lateral sclerosis (ALS), spinal cord tumours, syringo-myelia, etc. Thus, they need to be differentiated from HD becasuse it is non-progressive. Early and accurate diagnosis of HD is of prognostic value because it has clinical stability after a period of progression, compared to other debilitating diseases like motor neuron disease.
Magnetic resonance imaging (MRI), particularly in the flexion position, can help in the accurate diagnosis of HD and differentiate it from its mimickers. Loss of attachment (LOA) of the posterior dura mater on flexion MRI with enhancement of epidural venous plexus on post-contrast MRI is considered diagnostic of HD [1,5,6,9-11]. Flexion MRI also shows increased inter-segmental angle of flexion along with increased ROM in HD patients compared to the normal population [8,12]. The LOA of the posterior dura can also be picked up on neutral-position MRI. Careful evaluation of neutral-position MRI for this LOA, T2 hyperintense signal, asymmetrical atrophy of the lower cervical cord, and loss of lordosis may allow the radiologist to include flexion-position MRI, which is the gold standard for the diagnosis of HD [9,10,13,14].
Although the incidence of this disease is reported to be high in the Asian population [15,16], studies have also shown HD to be present in European, Middle Eastern, and North American populations [17,18]. We feel that the global burden of HD might be much more than is reported. This might be due the fact that only non-flexion (neutral position) MRI is performed in most of the patients due to unawareness amongst clinicians and radiologists about this disease.
Even though some studies have reported HD, the lite-rature is still sparse globally, especially from the develo-ping world. Hence, in our study, we explain the MRI features of HD in the northern Indian population done in both flexion position and neutral position on a 1.5 Tesla MRI machine. The purpose of our study was to evaluate the imaging features of Hirayama disease in both neutral and flexion MRI with utility of the inter-segmental angle of flexion (which is representative of the range of flexed motion).
Material and methods
We retrospectively reviewed images of patients with clinical diagnosis of HD based on their signs and symptoms, referred for MRI of the cervical spine, by searching our database and PACS for the keywords “Hirayama” and “flexion MRI” between January 2016 and March 2020. Institutional ethical clearance was not necessary because this was a retrospective study and no ionizing radiation was involved. Informed consent was waived. Twenty patients met the initial search criteria for the clinical diagnosis of Hirayama disease, and the MRIs of these patients were evaluated.
Inclusion criteria: Patients with unilateral or asymmetrical atrophy of the forearm and/or hand muscles with clinical diagnosis of HD.
Exclusion criteria: Patients who had a clinical diagnosis of HD but had an alternative diagnosis on MRI, and patients in whom flexion MRI was not done, were excluded from the study.
MRI protocol
All MRI scans were performed on a 1.5 T (Phillips Achieva) MRI scanner in both in neutral and flexion positions of the neck with a standard protocol. The following sequences were performed:
In the neutral position: T1W in the axial and sagittal planes (Spin Echo, TR/TE of 400 ms/80 ms) and T2TSE images in sagittal and axial planes (Turbo SE, TR/TE of 3000-4000 ms/110 ms). T2 FFE images were taken in the axial plane (gradient echo, TR/TE of 360 ms/9 ms, Flip angle of 25°).
In the flexion position: The neck was flexed by keeping a cushion under the cervical spine adjusted to the patient’s comfort level. T2W images were taken in both sagittal and axial planes with the same parameters as in the neutral position. Axial images were taken from C4 to T1 level. Post-contrast T1W fat sat images were taken in the sagittal and axial planes (TR/TE of 400 ms/80 ms) after intravenous injection of gadolinium-based contrast in a dose of 0.1 mmol/kg.
The slice thickness was 3 mm for all the sequences with a 0.3 mm gap.
Image analysis : All the images were retrieved on a workstation and analysed by 2 radiologists with 28 years and 8 years of experience, respectively. Disagreements were resolved by mutual consensus.
Neutral position MRI:
Cervical curvature was evaluated using the methods given by Guigui, Batzdorf, and Batzdorff [19,20]. Cervical lordosis was considered normal, straightened, or kyphotic on T2W sagittal images by drawing a line from the dorsal aspect of the C2 vertebral body to the inferodorsal aspect of C7. Cervical curvature is considered normal when no part of the dorsal aspect of the vertebral bodies crosses this line. Curvature is abnormal (straight or kyphotic) when part or all of the dorsal aspects of the vertebral bodies from C3 to C6 meet or cross through this line (Figure 1).
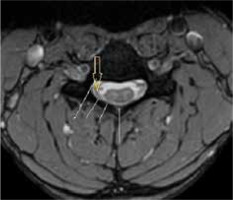
Axial T2TSE images were evaluated for loss of attachment (LOA) between the posterior dural sac and adjacent lamina on each side from level C4 to C6. The degree of separation was evaluated by a method given by Chen et al. [13]. Medial extent of the lamina was defined by the point of junction with the opposite lamina and laterally by a tangential line along the medial aspect of the pedicle. This was divided into 3 equal parts (Figure 2). LOA was considered present when the dural separation was greater than one-third of an adjacent lamina (Figure 3). This finding at a single level on one side was considered sufficient to score the presence of LOA [2].
Presence of lower cervical cord atrophy (from C4 to C7) was recorded. Loss of normal oval shape of the cord on axial images was considered as atrophy. A decrease in cord size in comparison with the normal cord above and below the affected level was defined as localized cord atrophy on sagittal MR images and confirmed on axial MR images [13]. Cord atrophy was recorded as symmetrical or asymmetrical.
Any T2 hyperintensity in the cervical cord was recorded.
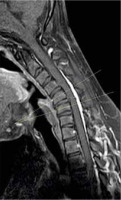
Figure 1
Sagittal T2W MRI image in a 23-year-old Hirayama disease patient showing kyphosis of the cervical spine. Posterior margins of the C3-C6 vertebrae are crossing the line drawn from inferodorsal aspect of C2 to inferodorsal aspect of C7 vertebral body
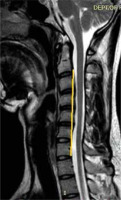
Figure 2
T2W axial image at the level of the pedicle in a normal person to show the method of determining the loss of attachment of posterior dura in the neutral position. The medial extent of lamina is at the level of the junction of 2 lamina and the lateral extent is the line along the medial side of the pedicle. This is divided into 3 equal parts to grade the degree of loss of dural attachment
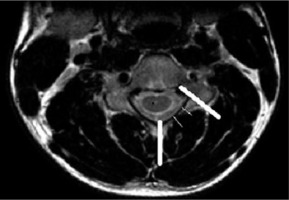
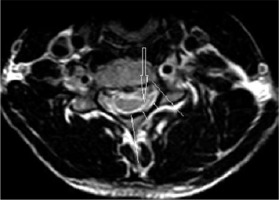
Figure 3
T2W axial image at the level of the pedicle in a 16-year-old male with Hirayama disease, showing loss of attachment of the dura (arrow), which is more than one-third, as shown by lines dividing the lamina into 3 equal parts
Flexion MRI:
Inter-segmental angles of flexion were recorded at C5-6 and C6-7 on T2W sagittal images by drawing a line along the middle borders of the vertebrae in the flexion position according to the method given by Schroder [17] (Figure 4).
T2W and post-contrast T1W images were evaluated as follows:
for forward displacement of posterior dura and any flow voids;
to ascertain any enhancement in the posterior epidural space on post-contrast images;
to ascertain whether forward displacement involved the whole (100%) or one side (50%) of the posterior dura;
to measure the maximum thickness of dural displacement in millimetres.
Results
Initial search and chart review
Twenty patients met the initial search criteria for the clinical diagnosis of Hirayama disease, and the MRIs of these patients were evaluated. Three patients were excluded from the study; 2 patients had syrinx in the cervical cord, while in the third patient flexion MRI was not available. Seventeen cases with a diagnosis of Hirayama disease were included in the study. The clinical features of these patients are summarized in Table 1. Most of the patients (16 out of 17) had a self-limiting course except for one patient whose motor symptoms deteriorated.
MR imaging evaluation
The median (IQR) age of the patients was 21 years (17-23 years). All patients were male.
Normal cervical lordosis was lost in 10 (58.8%) patients with HD, which included straightening in 7 patients (41.1%) and kyphosis in 3 patients (17.6%) (Figure 1).
Lower cervical cord atrophy was seen in all the patients, which was asymmetrical in 14 (82.3%) with laterality towards the right in 10 patients (Figures 5 and 6). Symmetrical cord atrophy was seen in 3 patients (17.6%). The extent of cord atrophy ranged from the C4 to the C7 vertebral level with involvement of the C5 to C6 level in all the patients.
Figure 5
T2W sagittal section of cervical spine in the neutral position, in a 22-year-old patient showing hyperintensity in the cervical cord (solid arrow) and atrophy of the lower cervical cord (open small arrows)
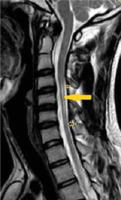
Figure 6
T2W axial image of cervical spine at the C5 level, in the same patient with Hirayama disease showing asymmetrical cord atrophy more towards the right side with hyperintensity in the cord (arrow)
Altered intramedullary signal in the form of T2W hyper-intensity was seen in only 6 patients (35.2%) (Figures 5 and 6).
LOA of posterior dura with anterior displacement was seen in all the patients (100%) on flexion MRI (Figure 7). LOA involved the whole of the posterior dura (bilateral) in 13 patients (76.4%) and only one side in 4 patients (23.5%). Maximum LOA was seen at the C6 level in 10 patients (58.8%), followed by C5-6 intervertebral disc level in 5 patients (29.4%), and C5 and C6-7 level in one patient each (5.8%). This LOA of the posterior dura was seen in 11 patients (64.7%) on neutral position MRI, as well (Figures 8 and 9). On evaluating axial T2W and T2 FFE images for LOA of posterior dura, mutual consensus was reached by 2 radiologists that it was better visualised on T2 FFE images (Figure 9). The mean thickness of this LOA on flexion was 3.7 ± 0.66 mm (mean ± 2SD).
Figure 7
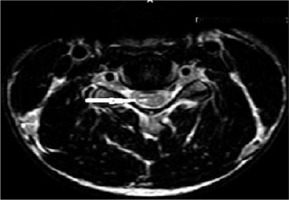
Sagittal T2W image in the flexion position in a patient showing anterior displacement of the posterior dura (white arrows). The flow voids are also seen in the posterior epidural space
Figure 8
T2 axial section in a 22-year-old male with Hirayama disease in the neutral position showing loss of attachment of the posterior dura (arrow)
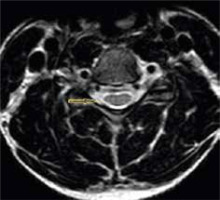
Figure 9
T2* axial section of the same patient in the neutral position showing loss of attachment from subjacent lamina, which is more than one-third and is better visualized than on T2W axial sections (arrow)
Posterior epidural flow voids were seen in 13 patients on T2W images on flexion MRI (76.4%) (Figure 7). Almost all the patients (16 out of 17) showed thick crescentic-shaped homogenous intense enhancement in the posterior epidural space in the flexion position (Figure 10). The imaging findings are summarized in Table 2.
Figure 10
Sagittal and axial T1W post-contrast image of the cervical spine in flexion position showing intense enhancement in the posterior epidural space (black arrow) extending from the C3 level to the T2 level (white arrows) and involving the bilateral sides (100% of the posterior dura is displaced forward)
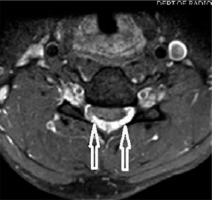
Table 2
MRI imaging findings of the Hirayama disease patients
The mean inter-segmental angle of flexion at C5 and C6 was 9.2°, and at C6 and C7 it was 6°.
Discussion
Conventional imaging studies (X-ray and fluoroscopy) have no role to play in picking up imaging findings of HD except for loss of cervical lordosis, which itself is a non-specific imaging finding and can be seen in many other patients. MRI is a non-invasive, non-ionizing, and convenient investigation for accurate diagnosis of HD. Neutral and flexion MRI views play a very important role in its accurate diagnosis, with flexion MRI being considered as the gold standard.
Flexion MRI shows the LOA, cord atrophy, and posterior epidural enhancement very well. On flexion MRI, all our patients showed forward displacement of the posterior dura, which is considered a hallmark of HD. This LOA of the posterior dura has been reported in various previous studies [5,18,21-24]. A study by Lehman et al. reported 100% specificity for the same, but with a lower sensitivity and false negative rates of LOA in a few patients [18]. These patients had a higher mean age (37.8 years), and it is believed that the findings of HD decrease with advancing age [1,15,18]. This forward displacement in the patients, measured as laminodural space (LDS) distance as stated by Boruah et al., can range from 3 to 9.8 mm, with a mean distance of approximately 5.99 mm [22]. However, our study showed this distance to be approx. 3.7 ± 0.66 mm (mean ± 2SD). This is lower than the distance measured in the study done by Boruah et al., which could be due to the larger number of patients included in their study. The most common site for maximum forward displacement of the dura in our study was at C6, seen in 58.8% of patients. The centre of displacement was also reported to be the C6 vertebra in the studies done by Hirayama et al. and Yin et al. [1,25]. On reviewing the axial sections for dural detachment from the adjacent lamina on flexion MRI in our patients, it was found to be bilateral in 13 patients (76.4%) and unilateral in 4 patients (23.5%). On searching the literature, we could not find any study on the evaluation of this imaging attribute in HD.
In another study done on HD, it was seen that the displacement of the posterior dura was also present in 46% of the normal population (controls); however, the extent of forward displacement was significantly less in them (0.99 ± 0.97 mm) compared to patients with HD (6.7 ± 0.78 mm). This forward displacement in controls was probably compensated by a corresponding increase in spinal canal volume, thus sparing the cord from compression [26]. However, the forward displacement in controls has not been reported by other studies [1,18,25]. Further research is required to study this imaging parameter in healthy subjects.
Crescentic homogenous enhancement was seen in the posterior epidural space on post-contrast flexion T1W images in almost all (93.7%) of the patients, which is in accordance with various studies [5,18,23]. Only one patient did not show enhancement: a follow-up case of HD aged 46 years. This can be explained by the fact that the findings of HD decrease with chronicity of the disease [1]. The enhancement has been attributed to the congestion of the posterior internal vertebral venous plexus [3]. There are 3 theories explaining the engorgement of venous plexus: 1) an anterior shift of the dura results in negative pressure in the posterior spinal canal, with resultant increased flow to the posterior internal vertebral venous plexus [6]; 2) the anterior internal vertebral venous plexus is compressed due to an anterior shift of the dura with resultant dilatation of the posterior internal vertebral venous plexus [5]; and 3) the venous drainage of the jugular veins is reduced in neck flexion, which compresses the venous return of the internal venous plexus [6]. Flow voids were seen in 76.4% of patients in the posterior epidural space on T2W images, which is in concordance with previous studies [10,11,13,22]. The aforementioned findings of forward displacement of the posterior dura, crescentic homogenous enhancement, and flow voids in the epidural space on flexion MRI are diagnostic of HD.
Another important imaging feature noted on flexion as well as neutral MRI is the lower cervical cord atrophy. This was seen in all the patients, with asymmetrical atrophy (82.3%) being much more common than symmetrical cord atrophy (17.6%). Similar findings of asymmetrical cord atrophy were also seen in other studies [1,10,13,23,27]. A “posterior epidural ligament factor” has been proposed by Shinomiya et al. [28] for this asymmetrical cord atrophy. These are the ligaments between the posterior dura mater and the ligamentum flavum, which prevent the separation of the posterior dura from the ligamentum flavum. These are abundant at the C1 and C2 levels and sparse at the C6 and C7 levels. An abnormally unequal distribution or lack of these ligaments at the lower cervical level may be the main cause of asymmetric cord compression. The extent of cord atrophy ranged from the C4 to the C7 level with involvement of the C5 and C6 level in all the patients in our study.
We also measured the inter-segmental angles of flexion on sagittal MRI at the C5-C6 and C6-C7 levels to see if the angle and ROM were greater in the lower cervical region in HD, because the surgical intervention is dependent on these findings. Maximum flexion was observed at the C5-C6 level followed by C6-C7 with a mean of 9.2° and 6o, respectively. As per Xu et al. also, the maximum flexion was seen at the C5-C6 level followed by the C6-C7 level, where the angles of flexion were 11.9° and 10.8°, respectively, whereas similar angles in controls were 5.4° and 5.6°, respectively [8]; our observations were similar except for the fact that we did not study angles of flexion in normal subjects. This shows that there is increased ROM in patients with HD, which could be an important factor related to the severity of the disease. Furthermore, because this increased ROM is amenable to surgical correction, which in turn can halt the progression of disease, mentioning the angle in the report becomes very important.
Neutral position MRI is equally important in picking up most of the aforementioned findings, which are clearly seen on flexion MRI. It is of utmost important for the radiologist to know the findings of HD on neutral MRI, so that based on his/her suspicion, he/she can then perform a flexion MRI and confirm his/her findings.
Asymmetrical lower cervical cord atrophy is the most important imaging feature on neutral MRI that can suggest the diagnosis of HD. LOA can also be seen on neutral MRI. On evaluating neutral T2W axial images in our study, LOA was seen to be present in 64.7% of the patients. As per Chen et al. [13], LOA was the most effective finding in the diagnosis of HD in neutral position MRI, with sensitivity and specificity greater than 93.5%; however, Lehman et al. reported lower sensitivity (70%) of this finding [18].
Hyperintense signal in the cervical cord on T2W images was seen in 35.2% of our patients, unlike the study by Hassan et al. in which it was seen in 18% of the patients with HD [29]. Intramedullary high signal intensity can be present because of more severe ischaemic change or gliosis in the vulnerable areas, but its incidence is lower than that of localized lower cervical cord atrophy [5,17].
Loss of cervical lordosis has also been reported in the literature in patients with HD. In our study, loss of cervical lordosis was seen in 58.8% of patients in the form of straightening (41.1%) or kyphosis (17.6%), unlike Hassan et al., who found loss of cervical lordosis in 91% of patients in their study [29]. In spite of this, loss of lordosis, in particular cervical straightening, is a very non-specific finding that can be seen in a lot of patients.
Although HD is a self-limiting disease, early diagnosis is important because it can stop the progression of the disease by timely intervention with cervical collar or surgical procedures, compared to other diagnosis such as multifocal motor neuropathy or ALS, which are progressive diseases [29,30]. Knowledge of the aforementioned findings on flexion and neutral MRI can help in early and accurate diagnosis.
There are certain limitations of our study. The small sample size is one of the main limitations, which is because of the rare occurrence of this disease. Another limitation is the retrospective design of the study. There was no control group available for comparison purposes. Finally, the radiologists were not blinded to the clinical diagnosis, which could have resulted in some bias.
Conclusions
MRI in the flexion position is the gold standard for HD, which shows the hallmark findings of loss of posterior dural attachment, enhancement of posterior epidural venous plexus, presence of flow voids, and increased inter-segmental angle of flexion. Increased range of flexed motion in patients with HD must be reported because surgical intervention is dependent on it. Careful evaluation of neutral-position MRI for the loss of dural attachment (which can be a subtle finding), asymmetric lower cord atrophy, T2 hyperintensity, and abnormal cervical curvature is of utmost importance because it prompts the radiologist to include a flexion sequence in the MR examination to confirm the findings of HD. Thus, familiarization of imaging findings of HD on neutral and flexion MRI and adopting a standardized MR imaging protocol can help in prompt diagnosis and early rehabilitation of the patient.