Clinical presentation
The cervical (C) spine is frequently involved in many inflammatory arthropathies. The most common are autoimmune disorders from the first 2 groups of rheumatic diseases, which are connective tissue diseases (such as rheumatoid arthritis [RA] and juvenile idiopathic arthritis [JIA]) and spondyloarthritis (SpA), among which the C-spine is mainly affected by psoriatic arthritis [1].
RA is the most common type of inflammatory arthritis. The C-spine, after the hands and wrists and the feet, is the third most commonly affected anatomical area in 86% of patients. The C1-C2 joint is most frequently involved. In approximately 20-70% of patients, C-spine lesions develop 10-11 years after disease onset, but in some individuals they may occur early and lead to instability. This condition, if untreated, may cause myelopathy with neurological deficits [2].
In RA, damage is caused by synovial and bone marrow inflammation (osteitis) [3,4]. As the disease progresses, pannus may lead to ligamental laxity and tears with subsequent atlanto-axial subluxation in different directions. Moreover, bone marrow inflammation and synovitis may lead to cartilage and bone damage with subchondral cysts and erosion [4]. Massive erosions with complete ligamental destruction may cause posterior or vertical atlantoaxial subluxations with subsequent spinal cord myelopathy or brain stem compression, which are life-threating conditions [5]. In addition to the C1/C2 level, subaxial spinal involvement may include (pseudo)spondylolisthesis, spinous process erosions, and rarely, discitis. Regardless of the disease duration, some patients may develop apophyseal joint ankylosis, which is also a risk factor for spinal myelopathy and cervical radiculopathy [6].
SpA does not frequently involve the C-spine. This group includes seronegative diseases (negative rheumatoid factor), such as ankylosing spondylitis (AS), psoriatic arthritis (PsA), reactive arthritis (ReA), arthritis associated with inflammatory bowel disease (IBD-related arthritis), and undifferentiated SpA [7]. Human leukocyte antigen (HLA)-B27 antigen positivity is associated with a higher prevalence of SpA, and the risk of developing SpA in the presence of HLA-B27 antigen positivity is 20 times greater than in the general population [8]. AS involves the C-spine in advanced disease, while PsA tends to involve the C-spine early in the disease. The lesions are SpA-specific and include syndesmophytes, parasyndesmophytes, ankylosis, vertebral squaring, and ligamental calcifications. They may less frequently show RA features, such as C1/C2 involvement with subluxations or dens erosions, which are mainly seen in PsA [9].
Enthesitis plays a crucial role in the pathogenesis of SpA-related lesions, and in the spine, it is observed in the anulus fibrosus of intravertebral joints. Early changes include bone marrow oedema (BME) of the anterior vertebral edges, also referred to as corner inflammatory lesions (CIL), with subsequent erosions (Romanus lesions) and triangular sclerosis, which are also called shiny corners. In more advanced disease, vertebral squaring (i.e. remodelling of the anterior aspect of the vertebral body) may be seen. This lesion is presumed to be caused by chronic osteitis and repair [10]. Syndesmophytes are the anterior or lateral ossification of anulus fibrosus or ligamental structures. In advanced disease, they may lead to ankylosis and spinal immobility.
Ankylosis affects longitudinal, flavum, interspinous, and supraspinous ligaments. In advanced disease, ligamental calcification may be encountered, typically in the posterior longitudinal ligament [11]. BME and the fatty degeneration of vertebral corners visible on magnetic resonance imaging (MRI) may predict the formation of new syndesmophytes [12,13]. Additionally, spinal fractures mainly occur in advanced AS and are often caused by minor trauma. At the cervical level, spinal fractures may lead to spinal cord injury or even sudden death [14]. Parasyndesmophytes are thick and irregular asymmetric syndesmophytes that sometimes occur in PsA and are less frequently observed in ReA [9].
JIA is the most common inflammatory arthritis of autoimmune and autoinflammatory origin in children under the age of 16 years. It is divided into 7 subtypes: oligoarticular JIA, seropositive polyarticular JIA, seronegative polyarticular JIA, systemic-onset JIA (sJIA), enthesitis-related arthritis (ERA), juvenile psoriatic arthritis (JPsA), and undifferentiated JIA [15]. JIA is characterized primarily by peripheral joint arthritis, but the C-spine can be affected in up to 80% of patients, most commonly in those with a polyarticular course of JIA [16].
C-spine lesions include synovial inflammation with pannus formation and BME. The indolent process of leucocyte infiltration into the synovium and proliferation of the synovial tissue lead to a subacute type of inflammation, which may explain why pain is not the most conspicuous feature of the clinical presentation of JIA and is sometimes absent, particularly in oligoarthritis JIA [17]. The most striking features of JIA are early C-spine apophyseal joint ankylosis accompanied by bone growth developmental disturbances, such as vertebral or disc hypoplasia caused by chronic inflammation and long-term steroid use [18]. Erosions and subluxations, similar to those observed in RA, also occur [16,19].
The first-line imaging modality for RA, SpA, and JIA is radiography, both static and dynamic. However, MRI allows for a more precise diagnosis of C-spine lesions, especially in terms of early diagnosis and when there is brainstem, spinal cord, or nerve-root involvement [20]. Computed tomography (CT) is mainly used preoperatively. The aim of this article is to review imaging features in RA, SpA, and JIA, taking into consideration the different pathogenetic backgrounds of each of them.
Imaging techniques
Radiography
Classic radiography is relatively effective in the assessment of bone lesions and C-spine alignment. The most commonly used views include lateral and anteroposterior (AP) projections, with the latter used for alignment and Luschka joint assessment. In rheumatological settings, functional lateral projections are often requested by clinicians or neuro-orthopaedic surgeons (flexion, extension, and neutral views) to assess subluxations, especially at the C1-C2 level. The lack of functional views and reliance only on lateral neutral projection leads to failed diagnosis in almost 50% of cases [21].
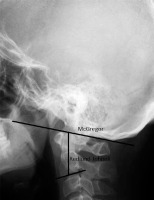
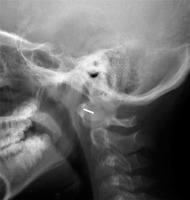
Another view used in rheumatology is AP projection with an open mouth, which is used to assess C1-C2 alignment and dens (Figure 1). However, in current practice, this view is rarely performed because it has been replaced by MRI and CT, which allow for a precise evaluation of this complex anatomical area. The significant limitation of plain radiography of the craniocervical junction is the superimposition of anatomical structures, which is especially common when rotational instability is present.
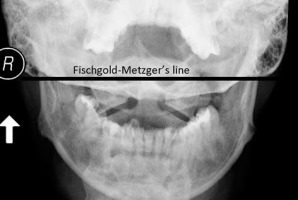
Figure 1
AP open mouth projection: Fischgold-Metzger’s line – example of measuring vertical subluxation
The evaluation of soft tissue in classical radiography is limited. However, in cases involving trauma, prevertebral soft tissue thickening can be assessed. The cut-offs for prevertebral thickness are 7 mm and 21 mm at the C2/C3 and C6/C7 levels, respectively [22]. In some patients, non-fused ossification centres are seen (e.g. os odontoideum), which need to be differentiated from fractures. In children, due to the laxity of the spine, false results concerning spondylolisthesis need to be considered.
Magnetic resonance imaging
Typical MRI protocols include sagittal T1- and T2-weighted sequences, T2 STIR (short tau inversion recovery), and axial T2-weighted images. Optionally, the coronal T2-weighted sequence can be used, primarily to evaluate lateral subluxation. Furthermore, sagittal post-contrast T1-weighted images can be used to assess active inflammatory lesions, mainly synovitis [23]. MRI is considered the gold standard for spinal cord and nerve-root imaging. Fluid-sensitive sequences with fat saturation are preferred for visualizing bone marrow oedema. MRI shows cysts, erosions of the dens or spinous processes, or vertebral endplates and spinal cord involvement in the C-spine. Functional MRI of the C-spine that includes flexion, extension, and neutral positions is possible, but it is not used in routine practice [24].
Computed tomography
For neuro-orthopaedic surgeons, CT of the C-spine is requested in almost all cases before surgery. With the possibility of 3-dimensional (3-D) reconstruction, CT perfectly outlines the complex anatomy of the cranio-cervical junction, including the atlanto-occipital joint, atlanto-axial joint, intervertebral joints, uncovertebral (Luschka) joints, and apophyseal joints. Multiplanar CT is superb in the visualization of anatomical variants, cysts, erosions, dens fractures, and subluxations, including the most challenging for radiography, the atlanto-axial level, as well as ankylosis. Although CT is superior in the assessment of soft tissue involvement when compared to radiography, it is still inferior to MRI, especially in the context of spinal cord and nerve-root imaging.
Imaging features of cervical spine pathologies
Subluxation
Rheumatological diseases, including RA, SpA, and JIA, have several disease-specific imaging features and share some common imaging features, including axial and subaxial subluxations and related spinal canal stenosis. This major cervical pathology, which is characterized by inappropriate vertebral alignment, can be divided into 2 categories based on the level of spine involvement: atlanto-axial at the C1-C2 level and subaxial at the C2-C7 level.
Atlanto-axial subluxation (AAS) is defined as a pathological alignment between the atlas (C1) and the dens of the axis (C2). It may occur in 3 dimensions: horizontal (anterior and posterior), lateral (right or left), and vertical (superior only). In anterior AAS, the distance between the posterior aspect of C1’s anterior arch and the anterior part of the dens (i.e. the anterior atlanto-dental interval [AADI]) exceeds 3 mm in adults (Figure 2) and 5 mm in children. Skeletal immaturity allows greater joint laxity in children, and the presence of cartilage causes a larger AADI. Posterior AAS is usually caused by a fracture or erosion of the dens (Figure 3). It strongly suggests spinal cord compression and is a predictor of neurological deficits [5]. In posterior AAS, when the distance between the posterior part of the dens and the anterior aspect of the posterior C1 arch (i.e. the posterior atlanto-dental interval [PADI]) is less than 14 mm, an urgent neurosurgical consultation is indicated. Lateral AAS is characterized by a displacement or asymmetry of C2 in relation to C1 that is equal to or greater than 2 mm. An open-mouth AP radiograph may be used to assess this pathology, but currently CT is more commonly performed to confirm the diagnosis [20].
Figure 2
Lateral radiograph of 59 y.o. female diagnosed with RA showing anterior AAS – 14 mm and dens erosions (between crosses). Bone loss and secondary osteoarthritis is also seen
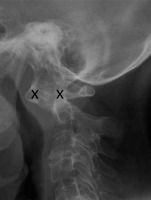
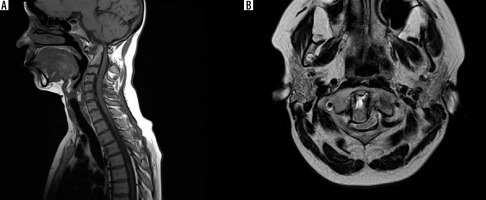
Figure 3
MRI of 44 y.o. female diagnosed with RA. A) T1-weighted sagittal scan showing anterior and posterior subluxation. Posterior atlanto-dental interval (PADI) is 6.5 mm. B) T2-weighted axial scan showing lateral and posterior subluxation with spinal cord compression by dens inflammatory tissue fills the space of C1/2 anteriorly to dens
Vertical AAS, also referred to as basilar settling, basilar invagination, or cranial settling, is defined as a vertical migration of the dens into the foramen magnum. It is usually caused by massive ligamental destruction or erosion at the occipito-atlanto-axial level. Basilar settling is almost always preceded by anterior AAS. Several methods are used to assess vertical AAS (Table 1; Figures 1 and 4-8). No method is ideal; however, if results obtained using Ranawat’s method, Redlunde Johnell’s line, or Clark’s method (or any combination thereof) are positive, the presence of vertical AAS is strongly suggested [25]. Approximately 6% of patients are misdiagnosed using these measurements in radiography. Therefore, a CT or an MRI should be performed when basilar settling is strongly suspected. It is worth mentioning that a phenomenon called pseudostabilization may occur with the presence and progression of basilar settling. In this case, the anterior AAS may decrease, and the dens seems to be stabilized on the anterior arch of the C1 axis [26]. Subaxial subluxation (SAS) is caused by progressive damage to the intervertebral, uncovertebral, and facet joints. The criterion for SAS is a greater than 3.5 mm displacement of adjacent vertebrae [27]. Other authors have suggested cut-off values of 2 mm and 4 mm for severe instability (Figure 9) [28].
Table 1
Comparison of different methods assessing basilar setting
| Method name | Description | Criterion for basilar setting |
|---|---|---|
| McGregor’s line [36] | Line between posterior aspect of hard palate and lowest part of occipital bone | Apex of dens is 4.5 mm above the line |
| Chamberlain’s line [37] | Line between posterior aspect of hard palate and opisthion | Apex of dens is 3 mm above the line |
| McRae’s line [38] | Line between basion and opisthion (foramen magnum) | Apex of dens is above the line |
| Wackenheim’s line [39] | Line drawn along the superior surface of clivus | Dens is located posterior to the line |
| Fischgold-Metzger’s line [40] | Line between mastoid processes on AP open mouth view | Apex of dens is above the line |
| Kauppi-Sakaguchi’s method [41] | Three lines are drawn: Upper line between superior edges of anterior and posterior C1 arch, lower line between inferior margins of anterior and posterior arch of C1, and middle line which connects midpoints of both arches. The superior facets of C2 (SFC2) are assessed. | Grade I: normal, SFC2 don’t cross lower line Grade II: abnormal, SFC2 lie between lower and middle lines Grade III: abnormal, SFC2 lie between middle and superior lines Grade IV: abnormal, SFC2 cross upper line |
| Ranawat’s method [42] | Line between central part of C2 pedicle and intersection of horizontal line drawn along the axis | Length below < 15 mm in males, < 13 mm in females |
| Redlund Johnell’s line [43] | Line between McGregor’s line and midpoint of caudal edge of C2 body | Length below < 34 mm in males, < 29 mm in females |
| Clark’s method [44] | Dens is divided into 3 equal stations on sagittal projection from superior to inferior | Anterior arch of C1 is adjacent to II or III station |
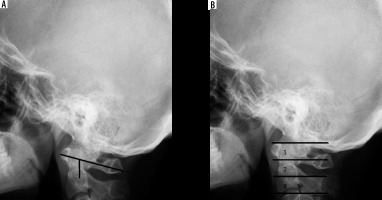
Figure 4
Lateral radiograph of healthy subject showing Chamberlain’s, McRae’s, and Wackenheim’s lines
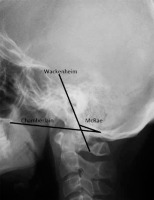
Figure 7
Lateral radiograph of healthy subject showing Kauppi-Sakaguchi method (curved line – superior facets of C2)
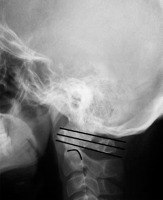
Figure 8
Lateral radiograph of 62 y.o. female diagnosed with RA showing anterior AAS and basilar settling (8.5 mm by McGregor’s method – black lines)
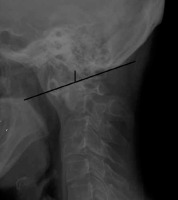
Figure 9
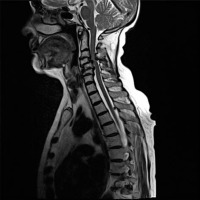
Sagittal MRI, T2-weighted sequence of 66 y.o. female diagnosed with RA showing multiple SAS at C3-C6 level
Cervical canal stenosis is diagnosed when the AP diameter of the spinal canal is below 10 mm (absolute stenosis). Relative stenosis is diagnosed when the AP diameter is 10-13 mm [29]. The preferred plane of measurement is lateral projection. The cervico-medullary angle (CMA) is the angle between lines drawn parallel to the anterior border of the upper cervical cord and the medulla oblongata. Normal values range between 135° and 175° [30], and values lower than 135° indicate the need for surgery when other clinical symptoms are present.
Rheumatoid arthritis
Imaging findings in RA include demineralization, bone marrow oedema, cysts, erosions, inflammatory pannus, subluxations, spondylolyses, ankylosis, and compression of the spinal cord, brain stem, or nerves. Younes et al. reported finding C1/C2 pannus formations in 62.5% of patients and dens erosions in 67.5% of patients with diagnosed RA and a disease duration of 2 or more years. Pannus leads to erosions and multidirectional subluxations, followed by ligament damage. Ligament lesions at the C1/2 are seen in approximately 65% of patients, and anterior AAS is the most common subluxation. Lateral AAS occurs in up to 20% of patients, while posterior AAS is observed in approximately 7%. Basilar settling (vertical AAS) and SAS occur in 20% and 15% of patients, respectively [31]. Posterior AAS is the most severe and life-threatening type of subluxation and results from severe dens destruction, along with ligamental damage. Dens erosions and cysts can be visualized by radiography, CT, and MRI, while MRI or CT is used for inflammatory pannus, and MRI is used for bone marrow oedema (Figures 10-12) [20].
Figure 11
Sagittal MRI, TIRM T2-weighted image of 40 y.o. female diagnosed with RA showing dens bone marrow oedema and paradonoid efussion
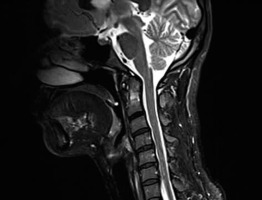
Figure 12
Sagittal MRI, T1-weighted sequence of 76 y.o. female diagnosed with RA showing extensive pannus formation and anterior AAS (4 mm; white line)
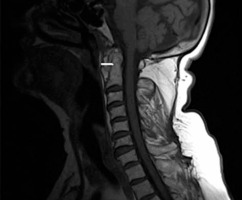
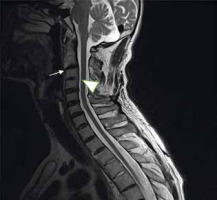
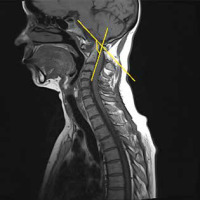
Cervical myelopathy can be caused by mechanical compression, vascular ischaemia, or both in a subluxated spine [32]. Patients with atlas erosion and a reduced distance between the dens and McRae’s line have a 5-fold increased risk of neurological manifestations [33]. In a previous study, occipital pain was present in all patients with basilar settling, while 80% had myelopathy, 53% had a history of loss of consciousness, 38% had signs of brainstem dysfunction, and 22% had lower cranial nerve palsies [34]. Therefore, it should be stressed that the severity of spinal cord compression is often underestimated in clinical exams. In a study conducted by Bundschuh et al., all patients with a cervicomedullary angle below 135° had either brain steam compression, myelopathy, or C2 nerve-root pain (Figure 13) [30].
Figure 13
Sagittal MRI, T2-weighted sequence of 44 y.o. female diagnosed with RA showing vertical and posterior subluxation (PADI < 14 mm) and cervicomedullary angle below 135° (yellow lines), which indicates brainstem compression
The following measurements are important criteria for spinal surgery: AADI > 6 mm, PADI < 14 mm, absolute cervical canal stenosis ≤ 10 mm, CME < 135°, and the presence of neurological symptoms (e.g. pain). Consensus has not been reached on whether surgery should be performed on patients with instability without clinical symptoms, which occurs in up to 15-50% of patients with RA [35]. In a retrospective study performed by Collins et al. [36], 50% of patients suffering from RA with no clinical signs had radiographic findings of cervical instability. In the symptomatic group, the most common manifestation was pain localized in the neck or the occipital, retro-orbital, or temporal regions [36]. Another study found that the apparent diffusion coefficient (ADC) in MRI was helpful in identifying cervical cord abnormalities in patients with anterior AAS but no spinal abnormalities on standard T1- or T2-weighted sequences. Patients with anterior AAS had higher ADC in the spinal cord at the C1 level, which presumable suggests early biochemical disturbances of the spinal cord [37].
Anterior AAS is diagnosed on radiographs but may not be confirmed on MRI due to the neutral position of the patient. However, subaxial myelopathy is confirmed by MRI and may be suspected on routine radiography when spinous process erosions, axial shortening of vertebrae, and spinal canal narrowing are present. Deterioration of subaxial myelopathy may be associated with the degree of anterior slippage, vertebrae or intervertebral space collapse, or destruction of the spinous process or apophyseal joint. Furthermore, younger patients and those with a longer disease duration, use of steroids, and a severe course of RA are at higher risk for subaxial myelopathy [38].
During clinical evaluation, Ranawat’s neurological scale has been associated with altered signals of the spinal cord on MRI [39]. Zhang et al. conducted a meta-analysis to study the prevalence of cervical spine involvement in the last 54 years (1960-2014). They found a decrease in the prevalence of anterior AAS but no changes in basilar settling or SAS. Thus, the progressive nature of C-spine involvement remains an issue [40].
Ankylosing spondylitis
Radiological involvement is present in up to 54% of AS cases and increases with age and disease duration [41]. Anterior AAS is present in up to 21% of cases, while vertical AAS is only found in 2%, and it has been found to correlate with high levels of C-reactive protein (CRP), peripheral arthritis, the degree of sacroiliitis, uveitis, and the use of biological treatment [12,42]. Maas et al. [43] found that 52% of patients with AS had syndesmophytes, and 25% had ankylosis of the facet joints. Interestingly, in this study, 26% and 13% of patients who underwent biological treatment developed new syndesmophytes and facet joint ankylosis, respectively, within 4 years. Destruction of the facet joints was linked to a longer disease duration and the presence of uveitis, psoriasis, or inflammatory bowel diseases, as well as high disease activity, a high modified stroke ankylosing spondylitis spinal score (mSASSS), the presence of syndesmophytes, and an increased occiput-to-wall distance measured at clinical assessment (the Flesche test) [43]. The progression of facet joint damage was associated with the presence of facet joint destruction early in AS and an increased occiput-to-wall distance [43].
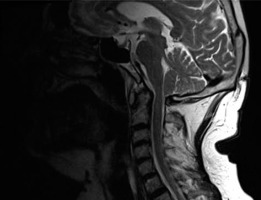
Ossification of the posterior longitudinal ligament is seen in 16% of cases and may lead to myelopathy [11]. Serious complications, including death, may result from vertebral fractures at the cervical level. They are associated with chin-to-chest deformity (evaluated during the clinical assessment) and are caused by excessive kyphosis. This type of fracture is the most common reason for surgical intervention at the level of the C-spine in AS [14]. Figure 14 presents syndesmophytes and ankylosis on MRI in a 52-year-old patient with a 24-year history of ankylosing spondylitis.
Psoriatic arthritis
Blau et al. categorized C-spine involvement in PsA into 2 heterogeneous groups: ankylosing and rheumatoid-like [44]. The first group is more commonly encountered and is characterized by the presence of ankylosis, syndesmophytes, and ligamental calcification. Rheumatoid-like PsA tends to be erosive with atlanto-axial subluxations [44, 45]. The C1/C2 level is affected in 30-75% of cases [44]. In a study conducted by Laiho et al., the most frequent lesion in the C-spine was bone ankylosis, accounting for 11% of patients, followed by anterior AAS in 8% of patients and vertical AAS in 5%. In PsA, thick and irregular asymmetric syndesmophytes, called parasyndesmophytes, are typically found in the thoraco-lumbar spine [9] but do not occur in the C-spine [46]. Patients with the polyarticular subtype of PsA are at the greatest risk of developing cervical lesions [47]. In a previous study, the duration of psoriatic arthritis and the presence of radiocarpal erosions were prognostic factors for C-spine disease, but no correlation was found regarding the severity of skin or nail disease [45].
Juvenile idiopathic arthritis
The most characteristic lesions in JIA include apophyseal joint ankylosis and vertebral hypoplasia. Other less specific features include dens erosions, anterior AAS, SAS, and ligamental calcifications. Ankylosis of apophyseal joints is the most common lesion, observed in up to 41% of patients [19,46]. Fusion typically begins at the C2/C3 level, and patients with early-onset JIA are at increased risk. It is common among patients with the polyarticular subtype of JIA and juvenile ankylosing spondylitis but has a similar rate of occurrence in seronegative and seropositive patients [19]. Regarding subluxations, anterior AAS has been observed in up to 33% of patients with the rheumatoid factor (RF) antibody, predisposing them to severe anterior AAS (Figure 15). Dens erosions were seen in up to 19% of cases, while SAS was seen in up to 7% [16].
Conclusions
Classic radiography is the first-line modality for C-spine imaging in inflammatory arthritis. MRI and CT allow for a more precise diagnosis by providing a detailed assessment of anatomy and soft-tissue pathologies. C-spine involvement in RA patients has a unique presentation, and it can be clinically silent, subtle, or nonspecific in the initial stages of RA, which may lead to severe cervical spine injury and peripheral joint disease. Furthermore, very few studies have focused on patients with SpA and JIA. Among SpA, mainly AS and PsA may affect the C-spine and lead to instability. In SpA, syndesmophytes, parasyndesmophytes, and bone ankylosis are common radiological findings. In JIA, early ankylosis is the most serious complication. C-spine imaging in patients with JIA is particularly important because it is difficult to assess clinically. Radiography has low sensitivity in children because it provides morphological and structural information more typical of the later phases of inflammation, so early detection with MRI is of utmost importance for prompt therapeutic intervention. Moreover, the early introduction of anti-tumour necrosis factor alpha (anti-TNFα) medication in the treatment of JIA patients with the severe form of the disease provides an opportunity to delay or prevent severe joint damage.