Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver, the sixth most common carcinoma in the world, and the fourth most common cause of cancer mortality [1,2]. HCC usually occurs in viral and non-viral cirrhotic liver. Hepatitis B and C viruses account for about 80% of HCC worldwide [3]. The diagnosis of HCC is based on typical imaging features in high-risk patients because lesion biopsy has several complications including haemorrhage, inadequate sampling, and seeding along the biopsy tract; thus, there is an increased need for fixed reproducible terms to standardize patient management [4].
Structured reporting with standardization of the radiological finding serves to increase the diagnostic accuracy of the report [5]. It also reduces the reporting variability and gives a definite answer for the clinician’s question. The American College of Radiology (ACR) has developed and updated the liver imaging reporting and data system (LI-RADS); a standardized structured reporting system for interpreting the imaging features of the hepatic focal lesion by different contrast-enhancing imaging modalities.
The LI-RADS lexicon imaging features are divided into major criteria encompassed of non-rim arterial hyper-enhancement (APHE), non-peripheral washout appearance (washout), and capsular enhancement and an optional ancillary feature; combinations of both major and ancillary features with lesion size and threshold growth are intrinsic steps for defining the LI-RADS category [6]. The goal of the LI-RADS scoring system is to establish a consistent terminology that facilitates the communication with clinicians, reducing misinterpretation, allowing definitive diagnosis of the hepatic focal lesion that fulfils the criteria of HCC, and risk stratification of other hepatic lesions that do not fulfil the HCC imaging criteria [7,8]. Although LI-RADS was first published several years ago, it has since undergone many improvements. The standardized reports should be a dynamic framework that will be refined and changed as new information and data are accumulated. More research is required to recognize whether the updated LI-RADS v2018 has enhanced inter-reader agreement.
The aim of the study was to assess the reliability and the inter-observer agreement of the major features of LI-RADS v2018 using dynamic magnetic resonance imaging (MRI) and to evaluate the reducibility of ancillary features and hepatic lesion size.
Material and methods
Patients
This study was conducted in our institution after the approval of our ethical committee and informed consent was waived. Adult (over the age of 18 years) patients with risk of developing HCC including patient with cirrhosis, chronic hepatitis B, or HCC were included. Contrast- enhanced dynamic MRI examination was performed for all patients in the period between August 2020 and March 2021. Ten patients were omitted from the study because they had HCC that was treated by local-regional treatment with no new observation in the dynamic MRI study, 5 patients because of motion artifact and low-quality scan, and 3 patients because a non-contrast MRI study was conducted. Finally, the study included 49 patients (35 males and 14 females), ranging in age from 29 to 82 years old.
Technique of dynamic magnetic resonance imaging examination
The MRI examination was done using a 1.5 T MRI (Philips Achieva scanner, Healthcare, Netherlands) with a body coil. FOV: 333 × 273 × 223 mm, slice thickness 8 mm, and gap: 0.7 mm. Sequences: breath-hold axial TSE T2-weighted imaging (TR/TE: 368/80 ms), breath-hold coronal single-shot T2-weighted imaging (TR/TE: 704/310 ms), in-phase axial GRE T1-weighted (TR/TE: 10/4.5 ms), out-of-phase axial GRE T1-weighted (TR/TE:10/2.2 ms), and respiratory-triggered axial DWI (echo-planar imaging; b-values, 0 and 800 s/mm2; TR/TE: 3062/62 ms). Post-contrast T1 fat-saturated THRIVE imaging was performed (late arterial 20-30 s; portal venous 60-90 s; and delayed 180-210 s) after injection of 0.1 mmol/kg gadolinium contrast media (TR/TE: 4/1.5 ms), flip angle 100, and slice thickness 2-3 mm.
Imaging analysis
The MRI examinations were first reviewed by a coordinator radiologist with 5 years of experience in liver imaging, who revealed 69 hepatic observations in 49 patients, for which a map was generated to allocate the segmental location of the observation, and then he assigned an ordinal number to each MRI series. The image series were independently interpreted by 2 radiologists with 4 and 3 years of experience in liver imaging, respectively, using a secondary workstation (Phillips Advantage Windows workstation). Each hepatic observation was evaluated according to the LI-RADS v.2018 lexicon [9], which included 4 major features; non-rim arterial hyperenhancement (APHE), non-peripheral washout appearance (washout), enhancing capsule, lesion size, and threshold growth, which could not be evaluated because this study prospectively evaluated retrospective data and it included only 1 MRI examination for each patient. The optional ancillary features are a diverse group including those that favour HCC as mosaic architecture, fat in mass, blood products in mass, or nodule-in-nodule architecture, those that favour malignancy in general as mild to moderate T2 hyperintensity and diffusion restriction, and those that favour benignity as marked T2 hyperintensity, iron in mass, and undistorted vessels. The observation was categorized as LR-1 for definitely benign (Figure 1), LR-2 for the probably benign (Figure 2), LR-3 for the intermediate probability of HCC (Figure 3), LR-4 for probably HCC (Figure 4), LR-5 for definitely HCC (Figure 5), LR-TIV (tumour in vein) for enhancing soft tissue in vein, with or without parenchymal mass (Figure 6), and LR-M for targetoid or non-targetoid mass lesion with radiological features suggesting non-HCC malignancy (Figure 7).
Figure 1
LR-1. Hepatic cyst. A) Axial T2-weighted image shows a high signal subcapsular hepatic lesion. B-D) No enhancement was seen in the arterial (B), portal venous (C), or delayed (D) phases
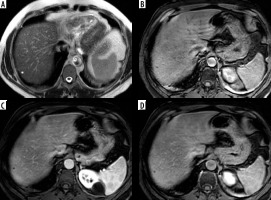
Figure 2
LR-2. Regenerative siderotic nodule in cirrhotic liver. A) Arterial phase shows a subcapsular hepatic focal lesion with no arterial hyper enhancement. B) Portal venous phase shows no washout or enhancing capsule. C) T2-weighted image shows a low signal hepatic lesion. D) Diffusion-weighted imaging shows no diffusion restriction in the hepatic lesion
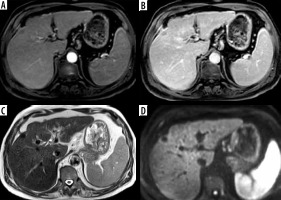
Figure 3
LR-3. A) Arterial phase image shows 2 hepatic focal lesions measuring about 1 cm with arterial hyper enhancement. B, C) Portal venous (B) and delayed (C) phases show no evidence of washout
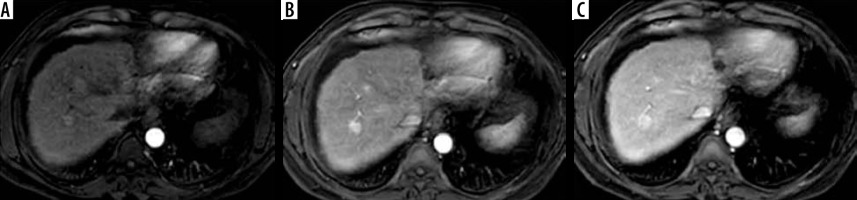
Figure 4
LR-4. A) Arterial phase image shows hepatic focal lesion at sub-segment VIII with arterial hyperenhancement. B) Delayed phase shows no evidence of washout with enhancing capsule. C, D) Diffusion-weighted imaging (C) and ADC (D) show no diffusion restriction. E, F) T2-weighted image (E) shows bright signal fat that shows loss of signal in the out of phase image (F)
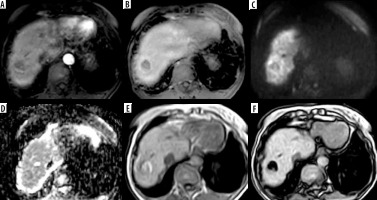
Figure 5
LR-5. A) Arterial phase shows a hepatic lesion in the left hepatic lobe with arterial hyperenhancement. B) The delayed phase shows washout with enhancing capsule. C) T2-weighted image shows an intermediate T2 signal. D) Diffusion-weighted imaging shows focal diffusion restriction. E, F) Foci of bright signal fat are seen in T1-weighted image (E) showing loss of signal in the out-of-phase image (F)
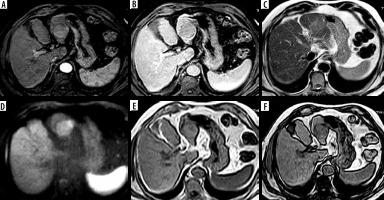
Figure 6
LR-TIV. A) Arterial phase image shows an infiltrative lesion with tumoral vein thrombosis in the right portal vein with arterial hyper enhancement. B, C) Portal venous (B) and delayed (C) phases show washout. D) Diffusion-weighted imaging shows diffusion restriction. E, F) Foci of bright signal are seen in axial T1-weighted image (E) showing loss of signal in out-of-phase images (F) denoting fat content

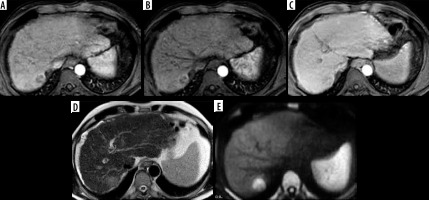
Figure 7
LR-M. A) Arterial phase image shows a hepatic focal lesion with rim arterial hyperenhancement and an enhancing centre. B) The portal venous phase shows no evidence of washout. C) The delayed phase shows no washout of the central enhancing area. D) T2-weighted image shows an intermediate T2 signal. E) Diffusion-weighted imaging shows diffusion restriction
Statistical analysis
Data were analysed using Statistical Package for Social Science (IBM SPSS statistics for windows, V. 22.0. Armonk, NY. USA). The qualitative data were described as number and per cent. To test the inter-reader agreement, Cohen’s kappa coefficient (k) test was performed for categorical and ordinal variables using cross-tabulation, and the interclass correlation (ICC) test was performed for the continuous variables. The 95% confidence interval was calculated. The κ and ICC tests were statistically significant as p < 0.05. Kappa agreement and ICC were interpreted as 0.01-0.20: slight agreement, 0.21-0.40: fair agreement, 0.41-0.60: moderate agreement, 0.61-0.80: substantial agreement, and 0.81-0.99: almost perfect agreement.
Results
Forty-nine patients with 69 hepatic observations were examined, 35 (71.5%) and 14 (28.5%) cases were male and female, respectively, and the mean patient age was 60.39 ± 12.42 years (range 29 to 82 years).
A substantial agreement was noted for APHE with κ = 0.796 (95% CI: 0.653-0.939; p < 0.001) and 89.9% agreement. The washout and the enhancing capsule also showed a substantial agreement with κ = 0.799 and 0.772, respectively (95% CI: 0.624-0.933 and 0.615-0.928, respectively; p < 0.001), and agreement of 89.8% (Table 1).
Table 1
Inter-reader agreement of the major features
In terms of ancillary features that favour HCC, there was almost perfect agreement for mosaic architecture, fat in mass, blood products in mass, and nodule-in-nodule architecture, with a κ from 0.818 to 1 (p < 0.001) (Table 2). A substantial agreement was noted for the ancillary features favouring malignancy in general, with κ = 0.754 and 0.718 (p < 0.001) for mild to moderate T2 hyperintensity and restricted diffusion, respectively (Table 3).
Table 2
Inter-reader agreement of ancillary features favouring hepatocellular carcinoma
Table 3
Inter-reader agreement of ancillary features favouring malignancy in general
As regards the ancillary features favouring benignity, an almost perfect agreement was noted for marked T2 hyperintensity and iron in mass with κ = 1 (p < 0.001), but substantial agreement was found between the 2 readers for undistorted vessels, with κ = 0.766 (p < 0.001) (Table 4).
Table 4
Inter-reader agreement of ancillary features favouring benignity
The Cohen’s κ coefficient showed a substantial agreement for the final LI-RADS category with κ = 0.651 (95% CI: 0.525-0.776; p < 0.001) and agreement of 70.9% (Table 5); the weighted κ showed higher yet still substantial agreement with weighted κ at 0.786 (95% CI: 0.697-0.876; p < 0.001). An almost perfect agreement was detected for LI-RADS TIV with κ = 0.818 (95% CI: 0.620-1; p < 0.001) and agreement of 95.7%.
Table 5
Inter-reader agreement of the final LI-RADS category
| Major feature | Reader (1) | Reader (2) | Matched cases | κ | 95% CI | p- value | Percent agreement |
|---|---|---|---|---|---|---|---|
| LR-1 | 6 | 8 | 4 | ||||
| LR-2 | 13 | 14 | 9 | ||||
| LR-3 | 18 | 14 | 13 | ||||
| LR-4 | 9 | 2 | 2 | ||||
| LR-5 | 13 | 16 | 12 | ||||
| LR-TIV | 8 | 11 | 8 | ||||
| LR-M | 2 | 4 | 1 | ||||
| LI-RADS | 0.651 | 0.525-0.776 | < 0.001* | 70.9 |
The mean hepatic lesion diameter measured by reader (1) and (2) was 32.6 mm (± 36.2) and 33.1 mm (± 35.2), respectively, with an excellent correlation between the 2 readers as the ICC = 0.977 (CI 95%: 0.964-0.986; p < 0.001).
Discussion
The frequency of HCC is increasing all over the world. Viral hepatitis represents the most common aetiology of HCC [1]. Definite early diagnosis may allow curative treatment opportunities for patients. Due to biopsy-related complications, the diagnosis of HCC is strongly dependent on imaging modalities, with consequently increased radiologist responsibility for HCC diagnosis [10]. Radiological reporting standardization with a structured algo-rism is considered the new radiological era, which helps to give a discrete answer to the clinical question of concern with significant participation in the next step of patient management according to radiological imaging suspicion.
The LI-RADS was released by ACR in an attempt to standardize the imaging interpretation and reduce the variability among radiological diagnoses. Assessment of the inter-reader agreement has become of utmost importance in the case of HCC as the diagnosis, and hence the treatment-based decision is based on the imaging features with no need for lesion biopsy in every case [11-14].
In the current study, the inter-reader agreement of the major features, the optional ancillary features, and the overall LI-RADS category were assessed using the dynamic MRI imaging protocol and LI-RADS v2018, and it revealed substantial agreement for detection of APHE, washout, and the enhancing capsule. In APHE the hepatic lesion enhances after contrast injection more than the hepatic parenchyma, and its MRI signal become more intense. The non-rim arterial hyperenhancement is usually noted in HCC and the rim arterial hyperenhancement is usually noted in malignant lesions other than HCC as metastases and cholangiocarcinomas, so it is used as a feature for lesion assignment as the LR-M category [15]. The non-peripheral washout feature is characterized as hepatic focal lesion becoming hypo-enhancing with low signal compared with the background hepatic tissue, and it is best appreciated during the portal venous or delayed phase (extracellular phase). Peripheral washout is defined as a lesion with hypo-enhancing periphery relative to its centre and the surrounding liver parenchyma, and it is not typical for HCC or benign hepatic lesion, but it is mostly seen with other hepatic malignant lesions, particularly metastases and cholangiocarcinoma, so the peri-pheral washout feature assigns the hepatic lesion as LR-M [16,17]. The enhancing capsule appears as a peri-pheral enhancing rim with a smooth margin, which occurs due to retention of contrast material within extravascular connective tissue, so its appearance is pronounced in the portal venous or delayed phase [15,18,19].
Several previous studies have reviewed LI-RADS v2013 and v2014 using the dynamic MRI modalities. Lower reliability was recorded for the LI-RADS v2013 lexicon, the range of κ coefficient for APHE, washout and capsular enhancement was 0.51 to 0.67, 0.48 to 0.69, and 0.37 to 0.59, respectively [20-22], but higher reliability was noted for the LI-RADS v2014, and the kappa coefficient range was 0.51 to 0.91, 0.45 to 0.83, and 0.36 to 0.89 for APHE, washout, and capsular enhancement, respectively [10,23-26]. Other studies have reviewed the agreement of LI-RADS v2017. The study by Kierans et al. [27] reported slight to fair inter-reader agreement of major LI-RADS features, their study was conducted using 1.5 and 3T MRI units, and used both the extracellular contrast agent and hepatobiliary agent. The study by Min et al. [28] was conducted using a 3T MRI system, and they reported almost perfect agreement for major features.
To our knowledge, 2 similar recent studies reviewed the reliability of LI-RADS v2018. Ludwig et al. [29] reviewed the diagnostic performance and the interobserver agreement of LI-RADS v2018 using both triphasic CT and dynamic MRI in a retrospective study performed in 2 liver transplant centres. Moderate agreement was reported for APHE and washout with a κ of 0.6 and 0.55, respectively. Abdel Razek et al. [30] reported an almost perfect agreement for APHE, washout, and capsular enhancement, with a κ of 0.948, 0.949, and 0.848, respectively. The standardized report must be a dynamic process, updated and refined continuously according to radiological experience, clinical consensus, and data validation [31,32].
Substantial agreement was noted for the final overall LI-RADS category. Studies by Fowler et al. [23] and Schellhaas et al. [24] also reported substantial agreement for the final LI-RADS category, with an ICC of 0.68, and κ of 0.61, but the study by Ludwig et al. [29] revealed moderate agreement for final LI-RADS category, with a κ of 0.5. On the other hand, Abdel Razek et al. [30] reported almost perfect agreement, with a κ of 0.99. The reliability of the overall LI-RADS category was less than that of the major feature lexicon; further refinement and accurate explanation of image interpretation will improve the reliability as the experience and data validation accumulate. This study also revealed that the mean difference between the 2 readers’ final category was small with higher weighted κ (0.786) compared to non-weighted κ (0.651), reflecting good reproducibility of the LI-RADS lexicon.
LR-TIV was introduced into LI-RADS v2017, replacing the previous LR-5V, and it is characterized by enhancing soft tissue into vein whether a parenchymal mass was noted or not. The current study revealed an almost perfect agreement for detection of LR-TIV. Abdel Razek et al. [30] also reported almost perfect agreement for LR-TIV, and Ludwig et al. [29] revealed substantial agreement for this newly updated feature.
Although the detection rate of the ancillary features was significantly variable, this study revealed almost perfect agreement for the ancillary features favouring HCC, and substantial to almost perfect agreement for other ancillary features. Min et al. [28] reported substantial to almost perfect agreement for some of the ancillary features. The ancillary features could be used to enhance lesion characterization, increase radiologist confidence for diagnosis, or adjust the LI-RADS category by no more than 1 category, with the exception of the LR-5 category [15,18]. An abbreviated MRI protocol without administration of contrast media could be used for surveillance of high-risk patients using the ancillary features for diagnosis of hepatic malignancy and HCC, especially in patients with severe renal impairment.
The current study revealed almost perfect agreement for hepatic lesion diameter measurement; similar agreement for the diameter measurement was also noted in many studies [20-22,30]. However, Sevim et al. [10] revealed less agreement for diameter measurement (ICC = 0.676). The precise diameter measurement is an important step in the LI-RADS lexicon because the final LI-RADS category is dependent on the lesion size.
The structured reporting system has many advantages, providing a definite clear language between radiologists, clinician, and patients, facilitating the communication between them, and providing comprehensive and consistent reporting by defining the major and ancillary features compared to free-text non-defined reporting. To standardize imaging interpretations, ongoing education with experience accumulation and revised and more comprehensive definitions may be needed.
The limitations met in this study were as follows: first, the sample size was somewhat limited. Schellhaas et al. [24] conducted their study in 50 hepatic lesions, but the current study included 69 hepatic lesions. Second, the inter-reader agreement of threshold growth was not determined. Third, the inter-reader agreement of the LI-RADS lexicon after HCC locoregional management was missed in this study, so a further study evaluating the LI-RADS treatment response with the implementation of functional imaging may be of value. Lastly, inter-modality agreement between computed tomography and MRI was not performed in our study.


