Introduction
The coronavirus disease 2019 (COVID-19) pandemic became the impulse to focus on messenger RNA (mRNA) vaccines’ wider research. Although this type of vaccine technology may seem new compared to the history of conventional vaccines, it has been investigated during the last 30 years on several viruses such as influenza, rabies, CMV, and Zika [1-3]. Pfizer-BioNTech and Moderna COVID-19 vaccines have received emergency use autho-rization from the Food and Drug Administration and European Medicines Agency due to following up. Both contain particles of mRNA that after injection instruct our immune cells to produce a spike protein, a non-irritant surface protein unrepeated by any other virus except SARS-CoV-2. This mentioned protein, recognized as an antigen, provokes an immune response with the production of T-lymphocytes and B-lymphocytes targeted to eliminate this specific antigen and induce immunity against the same pathogen in the future. A few conventional vaccines based on attenuated viral vectors to evoke immunization are being either developed or have already been approved and are being used in the world. These are Oxford-AstraZeneca, Johnson & Johnson, Sputnik V, and the CanSinoBIO-Beijing Institute of Biotechnology. The Oxford vaccine has been registered in Europe. AstraZeneca’s monovalent COVID-19 vaccine consists of a single recombinant, replication-deficient chimpanzee adenovirus vector (ChAdOx1). It also encodes the SARS-CoV-2 glycoprotein S as mRNA vaccines. Following injection, it is locally expressed to stimulate neutralizing antibodies and immune cells, which is fundamental for protection against COVID-19 [4].
In most cases, a positive protective effect of vaccines is observed, but occasionally adverse reactions occur, most of which are transient and minor. Locally activated antigens initially accumulate at the injection site and then migrate to the draining nodes. This mechanism is responsible for postvaccinal lymph node enlargement [5]. COVID-19 vaccines are administered into the deltoid muscle, so adenopathy occurs primarily in the axillary and supraclavicular regions [6, 7]. While it may occur soon after receiving any vaccination, it is more common after those that induce a strong immune response, including mRNA vaccines. The aim of this article is to highlight the importance of keeping this fact in mind, especially among radiologists who are involved in the diagnosis of oncology patients, and the result of the examination is unilateral lymph node enlargement. Cancers that show a predilection for node metastasis in these regions include breast cancer, head and neck cancers, lymphomas, and melanoma of the back and upper extremities. Rather than performing a potentially unnecessary and expensive biopsy, this will bring us closer to proper diagnosis and effective treatment. It will also allow patients to avoid unnecessary anxiety.
What is known about adenopathy after COVID-19 vaccination?
Before the widespread use of the COVID-19 vaccine, immunization was thought to be a rare cause of mild reactive axillary lymphadenopathy. Analysing data from clinical trials of both mRNA vaccines against SARS-CoV-2 may lead us to conclude that they are highly immunogenic, and when comparing them with other known vaccines, both local and systemic reactions are observed in a higher percentage of patients. In clinical studies, enlargement of axillary lymph nodes on the ipsilateral side of vaccine administration has been reported [8, 9] and was the second most commonly reported local reaction to the Moderna COVID-19 vaccine, in 11.6% and 16.0% of recipients after the first and second dose, respectively. Noted clinical enlargement of the axillary and supraclavicular lymph nodes was described in 1.1% of study participants [8]. In a comparable randomized, placebo-controlled, phase III clinical trial of the Pfizer-BioNTech COVID-19 vaccine, the incidence of ipsilateral axillary and supraclavicular lymph node enlargement was 0.3% among vaccine recipients compared with < 0.1% in the placebo group. There was a lower rate of post-vaccine lymphadenopathy than in the Moderna study. This is most likely because only patient self-reported symptoms of axillary adenopathy were collected [9]. Therefore, Pfizer’s data probably underestimates the true incidence of vaccine-induced axillary lymphadenopathy [10]. AstraZeneca’s summary of the safety profile of the vaccine indicates lymphadenopathy as an atypical finding. It affected 0.3% of patients [4]. Nonetheless, even with the predicted low relative incidence of regional adenopathy, the huge global volume of vaccines administered and the need to immunize the largest possible population in the shortest possible time is the reason why we will start to see secondary effects in diagnostic imaging. Clinically, axillary oedema or adenopathy appear within 2-4 days after each dose and last on average 1-2 days (Moderna) and 10 days (Pfizer-BioNTech).
What is still not well known about adenopathy after COVID-19 vaccination?
There is still no conclusive information on the form, incidence, or duration of adenopathy on imaging studies concerning different doses, different vaccines, or previous exposure to the COVID-19 virus. There are no data on the size, abundance, location, and morphology of the involved nodes or differences in different imaging modalities [11].
Extrapolating from preliminary cases and other conditions, imaging studies that are more sensitive than physical methods may show changes over a longer period [6], especially 18FDG-PET, in which inflammatory activity can be detected even in nodes that are not enlarged [12].
The weight to be given to the management and further implications of specific imaging features (e.g. long axis/width dimensions, cortical morphology, fat cavity behaviour, Doppler signal, SUV) remains unknown [11].
Clinical examples
Included in the article are figures from 4 different authors, which deal with reports of reactions only after mRNA vaccines. This is a weakness of this review, but at the time of writing, clinical data on other types of vaccines were not available. The next section highlights the relevance of the oncologic dilemma in the era of COVID-19 vaccination and concludes by offering preliminary recommendations and a practice algorithm.
Discussion
Becker et al. propose following the principle that the vaccine should be given on the side opposite the primary tumour site, if applicable, and that both doses of vaccine should be given on the same side into the deltoid muscle.
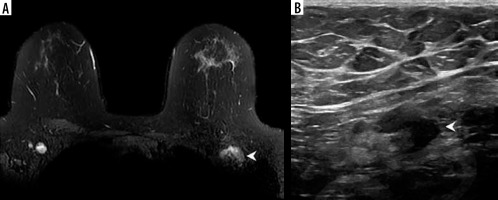
Breast imaging can be postponed until at least 4-6 weeks after the second dose of COVID-19 vaccination, “when it does not unduly delay care” [15]. Given their preliminary observations in which some nodes remain enlarged even after 4 weeks, a longer interval is desirable, as shown in Figure 1. This must be considered to the patient’s risk factors and overall context. Particular attention should be paid to modelling studies suggesting a high rate of missed cancer diagnoses due to the pandemic [16].
Figure 1
Example of vaccine-associated adenopathy in a 41-year-old female patient undergoing breast magnetic resonance imaging for follow-up of focal lesion. A) T2-weighted fat-saturated axial slice through the breasts and anterior chest, within 5 days of receiving COVID-19 vaccination in the left shoulder showing asymmetric left axillary adenopathy (3.0 × 1.7 cm, arrowhead) with preserved fatty hilum but irregular cortex. B) 6-week follow-up axillary sonography of the same patient demonstrates decreased size (2.2 × 1.1 cm) and some residual cortical thickening [Becker AS, Perez-Johnston R, Chikarmane SA, et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology 2021; 300: E323-E327. doi: 10.1148/radiol.2021210436]
Regarding the interpretation and reporting of imaging studies, they believe that vaccination information (date of each dose given, site of injection, including lateral location, and type of vaccine) should be included in all questionnaires before imaging unless it can be easily and reliably obtained from another source of medical records.
They propose that until more data are available, the committee recommends reporting morphological (e.g. size, number, shape), functional (e.g. MRI apparent diffusion coefficient value), and metabolic (e.g. standardized uptake values in PET) features of adenopathy found on imaging studies after vaccination. If clinically justified, follow-up ultrasound should be considered for further evaluation.
They emphasize that it is extremely important to inform patients about the expected side effects of COVID-19 vaccination and their significance [11].
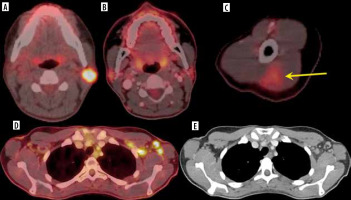
Özütemiz et al. note that vaccine-associated lympha-denopathy is not a new phenomenon in breast cancer imaging, but COVID-19 vaccine administration affects almost all imaging modalities, as presented in Figure 2.
Figure 2
A 32-year-old female. A) Axial fused 18FDG-PET/CT showed hypermetabolic biopsy proven intraparotid lymph node with metastatic malignant melanoma. B) Three-month follow-up axial fused 18FDG-PET/CT shows complete resolution of the neck mass following chemotherapy, (C) while the left arm shows hypermetabolic triangular shaped inflammation (arrow) at the COVID vaccine injection site. D) Axial fused images at the axilla level shows multiple new hypermetabolic lymph nodes. E) Axial contrast-enhanced CT demonstrates mild fat stranding surrounding the ovoid lymph nodes with preserved fatty hilum [Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncology Patients. Radiology 2021; 300: E296-E300. doi: 10.1148/radiol.2021210275]
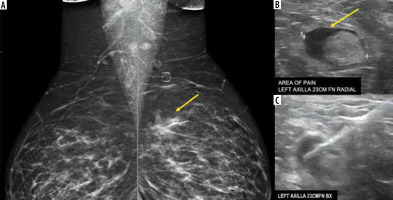
They obtained pathological confirmation of benign reactive lymphadenopathy secondary to vaccination in 2 cases (Figure 3). In the other 3, they did not confirm by histopathological examination that the lymph nodes were reactive. However, they consider that the recent administration of the vaccine is a very likely cause. In the absence of data on the duration of radiologically evident lymph node enlargement or recommended intervals between examinations, screening should temporarily be made dependent on the clinical context, especially in oncology patients in whom we cannot exclude a propensity for axillary lymph node enlargement [13].
Figure 3
A 38-year-old presented for evaluation of left breast and left axillary pain. A) Bilateral MLO views demonstrate an asymmetry (yellow arrow) in the left superior breast, which was biopsied as pseudoangiomatous stromal hyperplasia. B) Ultrasound evaluation of the left axilla demonstrates a lymph node with abnormally thickened cortex (yellow arrow). C) Ultrasound-guided core-needle biopsy for lymph node revealed reactive follicular hyperplasia [Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncology Patients. Radiology 2021; 300: E296-E300. doi: 10.1148/radiol.2021210275]
Hiller et al. decided to bring up this topic because of the significant number of cases of post-vaccination adverse reactions they encountered. They noted patients with lymphadenopathy of the supraclavicular, subclavian (Figure 4), and axillary regions on the same side as the injection. The appearance of new, especially non-painful, lymph node enlargement can indeed be very worrying. Also, they believe that currently patients presenting with lymphadenopathy must be asked if they have received a recent vaccine. Ultrasound examinations showed painful and non-painful lymphadenopathy. In cases of non-painful lymph node enlargement, their normal architecture and blood flow were preserved (Figure 4). In contrast, painful lymphadenopathy was associated with loss of normal anatomical node landmarks, liquefaction, and avascularisation, indicating node necrosis. Most significantly, regardless of clinical and sonographic appearance, all lymph nodes in the study series showed gradual resolution over up to 3 weeks. They also found that lymphadenopathy can occur after the first and/or second dose of the vaccine and appear after other, more common adverse reactions to the vaccine have resolved [14].
Figure 4
A) Focal swelling in the left infraclavicular area that prompted the study (arrow). B) Ultrasound image of the area demonstrating enlarged lymph nodes with a slightly hypoechoic cortical layer but with preserved hilum and blood supply [Hiller N, Goldberg S, Cohen-Cymberknoh M, et al. Lymphadenopathy Associated With the COVID-19 Vaccine. Cureus 2021; 13: e13524. doi: 10.7759/cureus.13524
Mitchell et al. stated that although the lymphatic pathways from the arm drain mainly to the axillary nodes, as shown in Figure 5, there are also connections to the supra-clavicular nodes in the lower neck. They also noted lymphadenopathy associated with COVID-19 vaccination, mimicking the Virchow-type node pattern. They suppose that with vaccination in the general population, such patients may be referred to neck tumour clinics [17].
Figure 5
A 42-year-old female with unilateral left axillary adenopathy noted 13 days after receiving the first dose of the Moderna COVID-19 vaccine in her left upper extremity. A) Grey-scale and (B) colour Doppler images of an enlarged left axillary lymph node with diffuse cortical thickening (arrows). Unremarkable right axilla was documented (C) [Mehta N, Sales RM, Babagbemi K, et al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging 2021; 75: 12-15. doi: 10.1016/j.clinimag.2021.01.016]
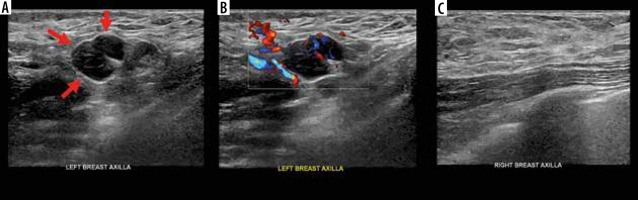
Edmonds et al. point out that further clinical data collection is needed to optimise the assessment and management of suspected post-vaccinal axillary lymphadenopathy on breast MRI. According to them, it will take at least several more months to obtain the necessary data. In the meantime, the dates of the COVID-19 vaccine doses are key in the medical record. They believe that in the current situation, isolated unilateral axillary lymphadenopathy detected on MRI on the side opposite the vaccine arm is most likely related to COVID-19 vaccine if it occurred within 4 weeks of any of the vaccine doses. In such cases, lymphadenopathy is graded BI-RADS 3 and a follow-up ultrasound is recommended 6-8 weeks after the second dose of vaccine. In addition, if clinically appropriate, a screening MRI scan may be scheduled 6-8 weeks after the second dose of vaccine to minimise the likelihood of detecting reactive lymphadenopathy requiring additional imaging studies [18].
Preliminary recommendations
Table 1 summarizes the recommendations created by Becker AS. The cornerstone of COVID-19 adenopathy management is efficient communication between patients, radiologists, and referring physician teams. Recommendations should be tailored to individual patients, based on risk classification and clinical context (including consideration of typical patterns of tumour spread and other factors such as tumour stage) [11].
Table 1
Summary of recommendations regarding adenopathy after vaccination for coronavirus disease 2019 (COVID-19) and radiologic imaging [Becker AS, Perez-Johnston R, Chikarmane SA, et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel. Radiology 2021; 300: E323-E327. doi: 10.1148/radiol.2021210436]
 |
In general, the essence of the recommendations is that COVID-19 vaccination should not be delayed because of the need for imaging studies in patients with a history of cancer or those undergoing cancer screening. The estimated mortality risk associated with COVID-19 infection is an order of magnitude higher than the estimated reduction in mortality achieved by effective cancer screening programmes in the general population [19]. Moreover, mortality from COVID-19 is likely to be worse in patients with cancer [20].
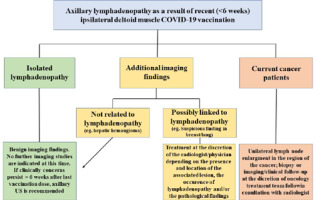
And another proposal for a simple algorithm (Figure 6) for axillary lymphadenopathy in the setting of recent (within 6 weeks) ipsilateral deltoid muscle COVID-19 vaccination across specialities.
Conclusions
New strains of this virus were identified only weeks before the start of mass vaccination against COVID-19, so it may only be a matter of time before new variants get out of control, necessitating the development and administration of new vaccines. It is also likely that booster doses will need to be introduced into the vaccination calendar. This cycle may become our daily routine, or perhaps it has already begun.
Overall, the radiologist needs to remember that recent COVID-19 immunization is a potential differential diagnosis of unilateral axillary adenopathy. This will allow appropriate recommendations for further management to be made. In addition, if clinically suitable and feasible, screening or, especially in oncology patients, follow-up imaging 6-8 weeks after the second dose of vaccine should be scheduled. This may minimise the likelihood of detecting reactive lymph node enlargement requiring further imaging studies [9] and reduce the number of false-positive recommendations for axillary lymph node biopsy, thereby minimising patient harm and cost.
The phenomena associated with COVID-19 vaccination deserve further scientific study. As more data are obtained on the expected time course of axillary lymph node enlargement after vaccination, the guidelines proposed herein will be refined.