Introduction
The optic nerve is a relatively small structure, with a total length of 50 mm, but there are numerous causes of optic nerve disorders, including: inflammation, tumours, vascular disorders, metabolic diseases, infections, drugs, systemic diseases, genetic diseases, and trauma (Table 1). The frequency of each disorder is relatively low, and it is difficult to differentiate between them in daily practice. Magnetic resonance imaging (MRI) is a common diagnostic imaging method for optic nerve disorders. However, abnormal findings for the optic nerve on MRI are similar and nonspecific for these diseases. Optic nerve enlargement with high signal intensity on T2-weighted images and contrast effect in the optic nerve are the main findings with optic nerve abnormalities. It is necessary to distinguish between abnormal findings around the optic nerve and clinical findings to differentiate these diseases [1,2]. In this paper, we review the imaging findings and clinical background of various diseases that cause optic nerve abnormalities.
Table 1
Differential diagnosis of optic nerve disorders
Anatomy
The optic nerve is commonly divided into four parts: intraocular, intraorbital, optic canal, and intracranial. In the orbit, the optic nerve is surrounded by meninges called the optic nerve sheath, which extends from the intracranial meninges to the eye. Cerebrospinal fluid exists between the optic nerve sheath and the optic nerve and is continuous with the intracranial arachnoid space [2]. The blood supply of the optic nerve comes mainly from the central retinal artery, which is a branch of the ophthalmic artery. The central retinal artery enters the optic nerve approximately 1 cm behind the eye [1,2].
Magnetic resonance imaging protocols
Magnetic resonance imaging protocols for optic nerve evaluation vary from report to report, and there is no single definitive protocol. Axial and coronal T1-weighted images without fat suppression, axial and coronal short-T1 inversion recovery (STIR) images, and axial and coronal post-contrast fat-suppressed T1-weighted images are common clinical MRI protocols for the optic nerve. MRI magnetic field strength of 1.5 T or 3 T is recommended, and the slice thickness should be less than 3 mm. The orbit and cavernous sinus should be included in both axial and coronal images [2].
Idiopathic optic neuritis
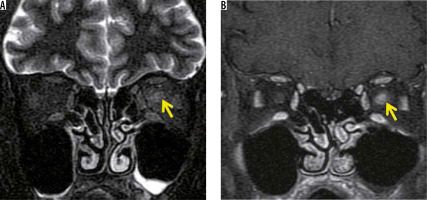
Idiopathic optic neuritis is an inflammation of the optic nerve of unknown cause. Idiopathic optic neuritis commonly affects women in their 20s or 30s. Typically, patients experience acute unilateral optic neuritis, resulting in vision loss and pain. Diagnosis of idiopathic optic neuritis requires the exclusion of demyelinating diseases such as multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMO-SD), and myelin oligodendrocyte glycoprotein antibody (MOG-Ab)-associated optic neuropathy [3]. Even if a diagnosis of idiopathic optic neuritis is made, patients often develop MS, especially in cases in which abnormalities are detected on brain MRI. In patients with optic neuritis, orbital MRI for the evaluation of optic neuritis and brain MRI for the negation of demyelinating disease are usually performed [4]. Idiopathic optic neuritis is evaluated by MRI, but the findings are non-specific. In the acute phase, the optic nerve is enlarged with high-intensity signal on T2-weighted or STIR images and has a contrast effect on post-contrast-enhanced T1-weighted image (Figure 1). On diffusion-weighted images, the optic nerve shows high signal with decreasing ADC value. In the chronic phase, the optic nerve is atrophied, with high-intensity signal on T2-weighted images, low-intensity signal on diffusion-weighted images, and poor contrast effect on post-contrast images [5].
Demyelinating disease
Acute disseminated encephalomyelitis (ADEM), MS, and NMO-SD are known inflammatory demyelinating diseases that cause optic neuritis. These disease names are heterogeneous in nature and the diseases are best viewed as ‘syndromes’ rather than specific disorders. In recent years, the detection of aquaporin-4 antibodies (AQP4-Ab) and MOG-Ab, which are the causative antibodies of inflammatory demyelinating disease, has been used for diagnosis, and the diagnosis of inflammatory demyelinating diseases is changing drastically [6-9].
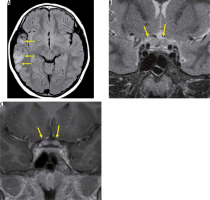
ADEM is an inflammatory demyelinating disease that is common in children and is thought to be an immune system dysregulation triggered by an infection or vaccine administration. Preinfection exists in 72-77% of patients with ADEM about one week before onset, and ADEM develops with symptoms such as: fever, headache, vomiting, visual abnormality, consciousness disturbance, and encephalitis. ADEM is usually monophasic, but when it recurs, it is classified as polymorphic ADEM, MS, or NMO-SD [9]. On brain MRI, a high- signal area on T2-weighted images is primarily observed in the subcortical white matter and is partially accompanied by a contrast effect. An abnormal signal is also found on the optic nerve, but it is indistinguishable from other types of optic neuritis (Figure 2) [10].
Figure 2
Optic neuritis due to acute disseminated encephalomyelitis. There are high-signal areas in the subcortical white matter on the right in the FLAIR image (A). The chiasm is enlarged with high signal intensity on the T2-weighted image (B). The chiasm is enhanced on the post-contrast T1-weighted image (C)
Multiple sclerosis is a disease that causes demyelination in the brain, spinal cord, and optic nerve, and it repeats cycles of relapse and remission during its course. MS often accompanies optic neuritis and is an important differential diagnosis from optic neuritis. MS is prevalent among women in their 20s to 30s and, unlike ADEM, it rarely develops before puberty [11]. MS is diagnosed by clinical and MRI findings according to the McDonald criteria, but the image findings of optic neuritis are not included in the McDonald criteria. Optic nerve lesions in patients with MS show a high signal on T2-weighted images and have a contrast effect, but the lesions cannot be distinguished from other types of optic neuritis. In brain MRI, a high-signal area is observed in T2-weighted images, mainly around the periventricular white matter. Patients who develop optic neuritis should undergo brain MRI to exclude MS [12].
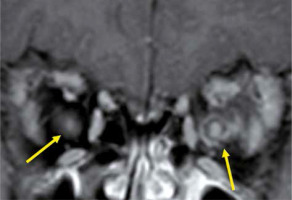
Neuromyelitis optica spectrum disorders, which are characterised by a positive test for AQP4-Ab, forms demyelinating lesions in the optic nerve and spinal cord. Previously, NMO-SD was regarded as a subtype of MS, but it was reclassified as an independent disease due to the discovery of AQP4-Ab. NMO-SD typically develops in women in their late 30s, with symptoms of transverse myelitis and bilateral optic neuritis. Diagnosis of NMO-SD is based mainly on AQP4-Ab, MRI, and clinical findings [8]. The optic nerve lesion in NMO-SD, like MS, is nonspecific on MRI (Figure 3), but exists for longer than that in MS [13]. On brain MRI, high signal on T2-weighted images is seen mainly in the dorsal side of the medulla, the fourth ventricle peripheries, the thalamus, and the hypothalamus, where the expression of AQP4 is high [14].
Figure 3
Optic neuritis due to neuromyelitis optica spectrum disorders. The left optic nerve shows a high signal and is swollen on the STIR image (A), and is enhanced on the fat-suppressed contrast T1-weighted image (B)
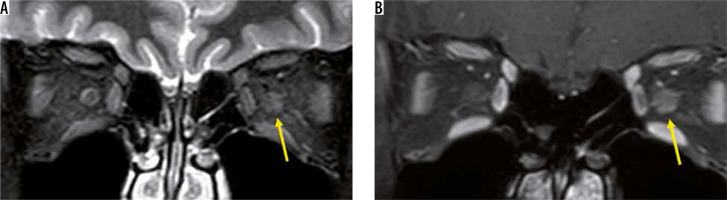
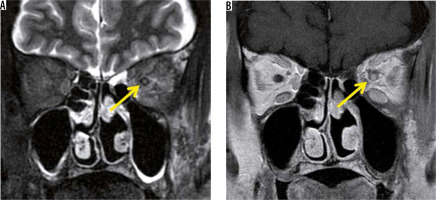
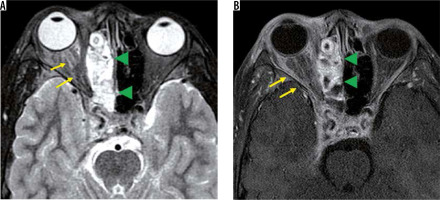
In recent years, MOG-Ab has also been found to cause demyelination in the central nervous system. Although MOG-Ab was first reported as a cause of AQP4-negative NMO-SD, MOG-Ab is also detected in patients diagnosed with idiopathic optic neuritis or ADEM [15]. Like other MRI findings in optic neuritis, the optic nerve lesion in MOG-Ab shows high-intensity signal on T2-weighted images with a contrast effect. The MOG-Ab-positive optic nerve lesion develops in the relatively anterior part of the optic nerve, whereas the AQP4-Ab-positive forms more posteriorly in the optic nerve. A contrast effect spreading around the optic nerve sheath is more suggestive of an MOG-Ab positive optic nerve lesion than an optic nerve lesion due to MS or an AQP4-Ab-positive optic nerve lesion (Figure 4) [16].
Hereditary optic neuropathy
Leber hereditary optic neuropathy and autosomal dominant optic atrophy are well-known hereditary neuropathies, but other hereditary optic neuropathies, such as congenital recessive optic atrophy, apparent sex-linked optic atrophy, and autosomal recessive chiasmal optic neuropathy, are all extremely rare [17].
Leber hereditary optic neuropathy is a maternally inherited disorder caused by a mitochondrial abnormality. Leber hereditary optic neuropathy typically affects men, |and patients usually develop subacute, progressive vision loss in their teens or 20s. Initially, the loss of vision is unilateral, but after several months vision in the contralateral eye also decreases. Unlike other types of optic neuritis, Leber hereditary optic neuropathy in its acute phase does not cause any abnormality on orbital MRI. However, in the chronic phase, the posterior optic nerve and optic chiasm enlarge with hyperintense signal on T2-weighted images [18].
Nutritional optic neuropathy
Nutritional optic neuropathy is a condition that causes bilateral loss of visual acuity due to nutrient deficiency. Nutritional optic neuropathy is rarely present in developed countries but may develop in individuals with extreme weight loss or an extremely unbalanced diet. Deficiency of vitamin B12 (cobalamin), vitamin B1 (thiamin), vitamin B2 (riboflavin), or folic acid can cause nutritional optic neuritis. Nutritional optic neuropathy is diagnosed based on a lack of nutrients in the blood. There are a few reports that MRI is useful in diagnosing nutritional optic neuropathy [19]. In patients with vitamin B1 deficiency (Wernicke’s encephalopathy), a high-signal area appears in the cerebral aqueduct, inside the thalamus, midbrain, and mammillary body, so brain MRI may be useful for diagnosis [20].
Toxic optic neuritis
Toxic optic neuritis is a neuropathy caused by the ingestion of toxic substances or by side effects of drugs. Numerous toxins and drugs are associated with optic neuritis. The mechanisms of optic nerve toxicity vary depending on the agent. Bilateral loss of vision is a typical symptom, and other neurological complaints may be present in some cases. Since most image findings of toxic optic neuritis are nonspecific, like other forms of optic neuritis, or are unknown, orbital MRI is performed to exclude other diseases that cause optic neuritis [21,22].
Radiation-induced optic neuritis
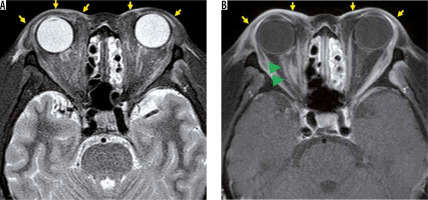
Radiation-induced optic neuritis is an essential complication of radiation therapy involving the anterior visual pathway; this neuritis can occur with a total dose of ≥ 50 Gy or a single dose of ≥ 10 Gy. Radiation-induced optic neuritis typically causes sudden unilateral vision impairment at around one year after radiotherapy, but occasionally occurs after three years. Within months after vision loss, the optic nerve in the irradiated area displays iso- or hyper-intensity on T2-weighted images, and contrast effect on T1-weighted images (Figure 5), but the contrast effect disappears within a few months [23].
Ischaemic optic neuropathy
Ischaemic optic neuropathy is the most common cause of unilateral optic neuropathy in patients over age 50 years. Ischaemic optic neuropathy is caused not by inflammation, but by impaired blood flow to the optic nerve. Ischaemic optic neuropathy is classified as either arteritic (mostly giant cell vasculitis) or non-arteritic (in which a shortage of blood flow to the optic nerve is suspected). Non-arteritic ischaemic optic neuropathy results from disease of the small vessels, but its exact cause remains unknown. The most common systemic disorders associated with non-arteritic ischaemic optic neuropathy are hypertension and diabetes mellitus.
Ischaemic optic neuropathy can be further classified into an anterior form, involving the papilla, and a posterior form, which does not involve the papilla. Anterior ischaemic optic neuropathy is diagnosed based on the presence of papilledema, but posterior ischaemic optic neuropathy is usually diagnosed by excluding diagnoses of other kinds of optic neuropathy, because it does not involve papilloedema [24].
MRI is useful for evaluating arteritic ischaemic optic neuropathy due to giant-cell arteritis. The optic neuropathy can be detected with minute contrast effects in the optic nerve by using enhanced black-blood 3D T1-weighted images [25]. In addition, MRI can be effectively used to diagnose temporal arteritis, by visualising wall thickening and contrast effect of the temporal artery [26]. Conversely, MRI is less useful in diagnosing non-arteritic ischaemic optic neuropathy because MRI can scarcely depict the optic nerve abnormalities. The main role of MRI in non-arteritic ischaemic optic neuropathy is to exclude other types of optic neuropathy [27].
Collagen vascular disease
Several collagen vascular diseases cause vasculitis in blood vessels of the optic nerve, resulting in visual impairment. Giant-cell vasculitis is the most common collagen vascular disease-causing optic neuritis, but Behçet’s disease, systemic lupus erythematosus (SLE), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) can also cause optic neuritis [28].
Behcet’s disease is a chronic, recurrent systemic inflammatory disease, in which the main symptoms are oral aphthae, vulva ulcers, skin lesions, and retinal choroiditis. In the eye, Behcet’s disease typically involves uveitis, but sometimes it affects the optic nerve. In MRI, enlargement of the optic nerve with high-intensity signal on STIR imaging and contrast effect around the optic nerve sheath (the so-called sunflower-like sign) is typical [29].
SLE is a chronic autoimmune disease that can affect multiple organ systems. Optic neuritis develops in about 1% of SLE patients and is usually accompanied by painful unilateral vision loss. The cause of optic neuritis is the autoimmunity by AQP4-Ab and ischaemia due to vasculitis. MRI shows optic nerve enlargement with contrast effect [30].
GPA is a type of necrotic, granulomatous vasculitis that spreads throughout the body, mainly in the upper respiratory tract and lungs; it is related to the antineutrophil cytoplasmic antibodies. Orbital involvement is common in GPA patients, occurring in approximately 50% of cases, and causes visual impairment due to intraorbital granuloma formation and scleritis. The intraorbital mass of GPA shows low signal intensity on T2-weighted images and is enhanced heterogeneously. GPA often accompanies sinus-wall thickening, and nasal-septal perforation also occurs (Figure 6) [31]. GPA also causes hypertrophic pachymeningitis, but it may progress to the optic nerve sheath and cause optic neuritis. MRI shows contrast effect on the thickened dura and the optic nerve sheath on the affected side, and a high-signal area exists in the optic nerve on T2-weighted images [32].
Figure 6
Granulomatosis with polyangiitis. On the T1-weighted image (A), a low-signal mass is visible in the left orbit (yellow arrow), and a heterogeneous-signal mass is visible in the ethmoid sinus (green arrowhead). The high T1 signal in the paranasal sinus reflects the exudate with micro-bleeding. On the STIR image (B), the left orbital tumour (yellow arrow) and ethmoid sinus (green arrowhead) show a mixture of low- and high-intensity signal. On the T1-weighted image (C), there is a nasal septum defect (*). Sinusitis with bleeding and a nasal septum defect suggest a diagnosis of granulomatous polyangiitis
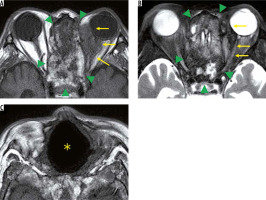
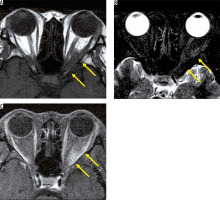
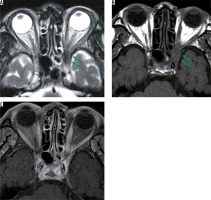
Eosinophilic granulomatosis with polyangiitis is defined as a systemic, necrotising small-vessel vasculitis, associated with asthma and with blood and tissue eosinophilia. EGPA often develops in the peripheral nerves, but sometimes it progresses to the central nervous system and the optic nerve. Patients with EGPA often suffer from optic neuritis or obstruction of the central retinal artery, resulting in impaired vision. MRI shows high-signal intensity in the orbit on STIR images in EGPA patients (Figure 7) [33].
Inflammatory disease
A variety of inflammatory or inflammation-like tumour masses can form in the orbit, all of which cause a decrease in visual acuity. Such known tumour masses include immunoglobulin G4 (IgG4)-related disease, sarcoidosis, Erdheim-Chester disease, and inflammatory pseudotumour.
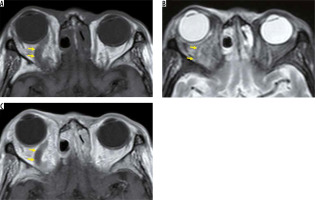
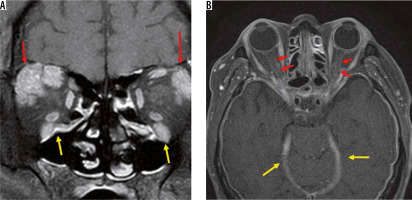
IgG4-related diseases are chronic inflammatory diseases in which blood IgG4 is elevated, forming a tumour that causes IgG4-positive plasma cells to infiltrate various organs. IgG4-related diseases are diagnosed according to imaging findings, elevated blood IgG4, and pathological findings. The orbital MRI findings in IgG4-related diseases are swelling of the lacrimal gland, intraorbital mass, enlargement of the extraocular muscle(s), and enlargement of cranial nerves, such as the suborbital nerve. Hypertrophic meningitis sometimes affects patients with IgG4 diseases, and it can spread to the optic nerve sheath (Figure 8). When IgG4-related hypertrophic meningitis in the optic nerve sheath presses on the optic nerve, visual function can be reduced [34].
Figure 8
IgG4-related disorders. Two cases of IgG4-related disease are shown. Bilateral lacrimal glands (red arrows) and infraorbital nerves (yellow arrows) are enlarged due to the IgG4-related disease on the contrast-enhanced coronal T1-weighted image (A). The dura (yellow arrows) and the bilateral optic nerve sheaths (red arrows) have been thickened and contrast-enhanced due to the hypertrophic meningitis on the contrast-enhanced, T1-weighted image (B)
Sarcoidosis is an inflammatory disease of unknown cause, which occurs in multiple organs. Uveitis and retinitis are frequent manifestation of sarcoidosis, but optic nerve lesions are uncommon. However, in cases of neurosarcoidosis, cranial nerve involvement is the most common presenting feature (55%), with the optic nerve being most commonly affected (33-75%). Optic nerve sarcoidosis usually occurs unilaterally, but it develops bilaterally in 31% of patients. With orbital MRI, the optic nerve shows a high signal on T2-weighted images, and contrast effect exists in the optic nerve and optic nerve sheath [35].
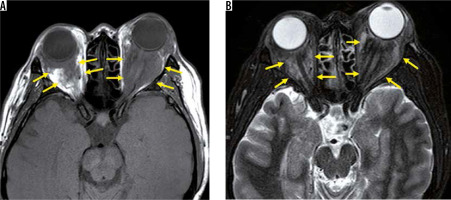
Erdheim-Chester disease is a rare non-Langerhans cell histiocytosis that is characterised by xanthomatous infiltration of tissues. Patients with Erdheim-Chester disease usually develop limb-bone pain in their 40s or 50s. Erdheim-Chester disease is characterised by bilateral and symmetric osteosclerosis of the diaphysis of the long bones on X-ray. The orbital lesions mainly show low-intensity signal on both T1- and T2-weighted images, and diffuse enhancement in post-contrast T1-weighted images (Figure 9). Although the mass pressures the optic nerve, vision deterioration is rare [36].
Figure 9
Erdheim-Chester disease. There is a low-signal-intensity mass in the bilateral orbital fat around the optic nerve on the T1-weighted image (A). The mass shows heterogenous signal on the STIR image (B)
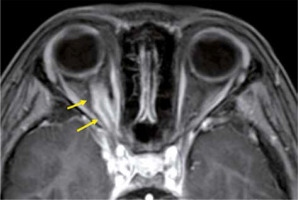
Inflammatory pseudotumour is a non-infectious inflammatory mass, without systemic disease, and is the most common type among painful orbital mass. The cause of inflammatory pseudotumour is unknown, and the diagnosis is made by exclusion, based on clinical and imaging findings. The symptoms differ depending on the lesion site, but when it is close to the optic nerve, it causes a decrease in visual function. Inflammatory pseudotumour occurs around the lacrimal gland, extraocular muscle peripheries, optic nerve sheath circumference, orbital apex, and so on. Inflammatory pseudotumours show low-intensity signal on both T1-and T2-weighted images with orbital MRI (Figure 10) [37].
Infection
A variety of bacterial infections, fungal infections, and viral infections can occur in the orbit. When inflammation due to infection reaches the optic nerve, visual function deteriorates. Orbital infections usually originate from sinusitis, especially from the ethmoid sinuses, leading to orbital bacterial or fungal infections. Bacterial infections of the orbit are common in healthy individuals. MRIs of patients with bacterial sinusitis-induced optic neuritis show paranasal sinuses and orbital fat with low signals on T1-weighted images; high signals appear on fat-suppression T2-weighted images. The optic nerve is surrounded by the mass and presents a high-intensity signal on T2-weighted images (Figure 11) [38].
Figure 11
Optic neuritis due to bacterial sinusitis. The mucosa of the right ethmoid sinus is thickened (green arrowheads) with high-intensity signal, and the right orbital fat and the optic nerve (yellow arrow) show high signal intensity. On fat-suppressed contrast T1-weighted image (B), the right ethmoid sinus (green arrowheads) and orbit (yellow arrow) are heterogeneously enhanced
Fungal sinusitis is histologically divided into invasive and non-invasive forms. Invasive sinusitis is rare and likely to occur in immunocompromised patients. Fungal optic neuritis is caused by invasive sinusitis and is similar to its bacterial counterpart, but sinus-wall thickening and orbital lesions are often limited. Furthermore, fungal infections of the paranasal sinuses and orbits show low signals in T2-weighted images and become obscure in fat-suppressed, T2-weighted images on MRI. In cases of optic neuritis due to fungal sinusitis, attention should be paid to the abnormality of the fat signal at the orbital apex (Figure 12) [39].
Figure 12
Invasive aspergillosis of the orbital apex. The orbital apex has low signal (green arrows), and the mucosa of the ethmoid sinus is mildly thickened on the STIR image (A). The fat tissue of the orbital apex (green arrows) has low signal intensity compared with the contralateral side on the T1-weighted image (B). It is difficult to detect the lesion on the post-contrast fat-suppressed, T1-weighted image (C)
Tuberculous optic neuritis occurs as tuberculous meningitis develops in the optic nerve and optic chiasm. In addition to the presence of meningitis, the ring-shaped contrast effect and swelling are recognisable in the optic nerve in cases of tuberculous optic neuritis. Brain MRI visualises hydrocephalus, meningeal hyperplasia, and tuberculoma, which are findings of tuberculous meningitis [40].
The optic nerve can also be affected by various infectious diseases such as Bartonella, cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, Lyme disease, syphilis, toxoplasmosis, toxocariasis, and varicella zoster virus, but there are no comprehensive reports about MRI findings in optic neuritis due to these infections [14].
Traumatic optic neuropathy
Traumatic optic neuropathy is a complication of head and orbit trauma, and can be divided into direct injury and indirect injury cases. Indirect injury is more common than direct injury, and the incidence of traumatic optic neuropathy is estimated to be 0.5-5% in cases of head trauma. Optic neuropathy due to indirect injury is a traumatic visual disorder with no abnormality in the optic nerve, diagnosed with ophthalmoscopic and image analysis such as CT and MRI. However, in optic neuropathy due to direct injury, image analysis can visualise bone fragments and foreign bodies that damage the optic nerve. CT is the first test method for traumatic optic neuropathy because it can evaluate the whole body at the same time and can depict most foreign bodies and bone fragments. MRI can visualise optic nerve damage as a high-intensity signal on T2-weighted images, but only plays a small role in diagnosing traumatic optic neuropathy [41].
Vascular abnormalities
Carotid cavernous fistula (CCF) is an abnormal communication between the carotid artery and vein in the cavernous sinus, due to trauma or the rupture of an aneurysm. Symptoms of CCF include ocular prolapse, red eye, bruit, and double vision, but visual acuity reduction due to high venous pressure also occurs. MRI findings from a CCF show no abnormality in the optic nerve, but they are often accompanied by dilation of the superior ocular vein and cavernous sinus (Figure 13). MR angiography is also used for diagnosis of CCF, but exclusion diagnosis of CCF is difficult when only using MRA, due to poor sensitivity. The diagnosis of CCF is usually based on angiography or CT angiography [42].
Figure 13
Carotid cavernous fistula. Dilation of the superior ophthalmic vein (arrows) on this T2-weighted image is a finding that casts doubt on the carotid cavernous fistula
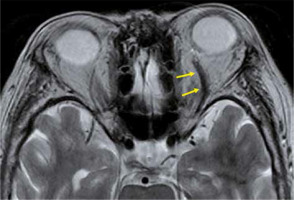
Superior ophthalmic vein thrombosis is a very rare disorder, which is caused by orbital infections and various kinds of inflammation. The superior ophthalmic vein is responsible for most of the venous perfusion of the orbit, and superior ophthalmic vein thrombosis causes protrusion of the eye, a decrease in visual acuity, and limitations of eye movements. Superior ophthalmic vein thrombus presents an enlarged superior ophthalmic vein with a high-intensity signal in the lumen on T2-weighted images. The vein wall is thickened with a contrast effect, but there is no contrast effect in the lumen (Figure 14). Cavernous sinus thrombosis is often accompanied by superior ophthalmic vein thrombosis [43].
Optic nerve and optic nerve sheath neoplasm
Of tumours arising from the optic nerve or optic nerve sheath, optic gliomas and meningiomas are common [44]. Optic gliomas are the most common tumours of optic nerve origin. Optic gliomas are common in children under the age of eight years, and most can be classified as juvenile pilocytic astrocytomas. Optic gliomas are often associated with neurofibromatosis type 1 (NF1) and are found in 20% of NF1 cases. Sporadic optic gliomas are more likely to be localised to the optic chiasm and are more symptomatic than NF1-associated optic gliomas. MRI can visualise the enlargement of the optic nerve and show a high signal on T2-weighted images, but the contrast effect of the tumour varies. There may be a high-signal area at the periphery of the tumour on T2-weighted images, which is highly suggestive of optic glioma [44,45].
Optic nerve sheath meningioma is a type of meningioma that spreads along the meninges of the optic nerve sheath. Ninety per cent of optic nerve sheath meningiomas develop from the intracranial meninges, with only 10% originating from the optic nerve sheath. Because the optic nerve sheath is narrow, most optic nerve sheath meningiomas compromise visual function. MRI displays a soft-tissue mass along the optic nerve sheath, and the contrast effect along the optic nerve sheath can be recognised on post-contrast-enhanced, T1-weighted images. The two linear contrast effects along the optic nerve on contrast-enhanced, axial T1-weighted images are called the “tram-track sign” (Figure 15), but this finding is not specific to optic nerve meningiomas because it is also found in cases of optic perineuritis. CT aids in diagnoses by depicting calcification in the tumour [44].
Orbital tumour
The optic nerve is compressed by various tumours, which results in visual function reduction, particularly with tumours at the orbital apex. Tumours that compress the optic nerve include primary orbital tumours, direct tumour invasion from an adjacent structure, and metastatic orbital tumours. Of orbital tumours, lymphoma, cavernous haemangiomas, and schwannomas frequently occur, and, even if they are benign, they press on the optic nerve [44].
Lymphoma is the most frequently occurring of intra orbital tumours and is common in people over the age of 60 years. On MRI, lymphoma is often seen as an ill-defined mass, but sometimes it is a smooth, circumscribed mass. Lymphoma may spread along the existing structures, such as the optic nerve, and this finding is characteristic of lymphoma (Figure 16) [46]. Cavernous haemangiomas are not true tumours but are the most common orbital masses in adults. On MRI, cavernous haemangioma presents a smooth border with hyperintensity on T2-weighted images. Visual abnormalities due to cavernous haemangiomas are rare, but they can cause compressive optic neuritis at the orbital apex. Schwannomas are rare in the orbit, but they arise from the trigeminal, oculomotor, trochlear, and abducens nerves. On MRI, a schwannoma is a well-defined mass that develops along the nerve. A schwannoma is a mixture of components of Antoni A areas, characterised by rich cellular areas, and Antoni B areas, characterised by poor cellular areas, and it exhibits a complicated signal on T2-weighted images (Figure 17). Although visual performance rarely deteriorates, it can if the optic nerve is compressed [44].
Figure 16
Lymphoma. An ill-defined low-signal mass (yellow arrows) is located around the left optic nerve on the T1-weighted image (A). The mass has low signal on the STIR image (B) and contrast effect on the post-contrast, fat-suppressed, T1-weighted image (C)
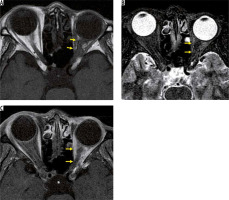
Figure 17
Schwannomas of the oculomotor nerve. A dumbbell-shaped tumour exists from the cavernous sinus to the orbit (yellow arrows). The tumour periphery has high signal intensity, and the interior is iso-intense with the brain on the STIR image (A). The inside of the tumour is enhanced on the post-contrast, fat-suppressed, T1-weighted image (B)
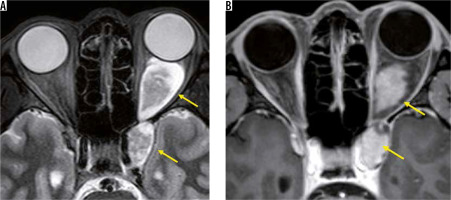
Paranasal malignancies are commonly accompanied orbital invasion. In many cases, the infiltration of the sinus by malignant tumours remains in the orbital periosteum, and it is rare that an optic nerve injury occurs at the beginning. However, local recurrence of the tumour often invades the optic nerve [47].
Metastatic tumours account for 1-13% of orbital tumours, and 16-23% of patients develop vision loss. Breast cancer, lung cancer, and prostate cancer are common sources of orbital metastases, but their frequency differs depending on the demographics and country of origin of the study. The image findings of metastatic tumours are nonspecific except for forming mass in the orbit (Figure 18), and the history of malignant tumours is important [48].
Figure 18
Optic canal metastasis from breast cancer. The right orbital apex has low signal (yellow arrows) compared with the contralateral side on the T1-weighted image (A). The right orbital apex is heterogeneously enhanced (yellow arrows) compared with the contralateral side on the fat-suppressed, post-contrast, T1-weighted image (B). As a result of the whole-body assessment, the tumour was diagnosed as an optic canal metastasis from the patient’s breast cancer
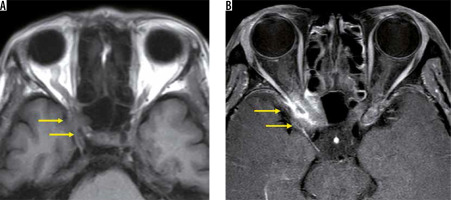
Compressive optic neuropathy
Compressive optic neuritis is a form of neuritis that occurs when the optic nerve is compressed by a mass. This section deals with compression that is not caused by tumours or inflammatory masses.
A mucocele is not a tumour, but it forms encapsulated mucous cavities in the paranasal sinuses. As the mucocele increases, it progresses to the orbit and compresses the optic nerve. When the mucocele occurs in the Onodi cell surrounding the optic nerve, or the anterior floor processes, it is close to the optic nerve and often causes acute vision loss. A mucocele is a smooth-margin mass that causes bulging in the surrounding bone, and in many cases it is easy to distinguish from malignant paranasal sinus tumours. On MRI, a mucocele presents high-intensity signals in both T1-weighted images and T2-weighted images without contrast effect due to fluid containing a high level of protein (Figure 19) [49].
Figure 19
Mucocele in the Onodi cell. On the T1-weighted image (A) a high-signal mass with a clear border (yellow arrow) is located inside the optic canal. The surrounding bone is expanded and thinned on the axial CT (B). The optic canal is narrowed by the tumour (yellow arrow) on coronal CT (C)
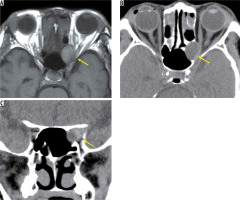
Thyroid ophthalmopathy is an orbital condition that has a strong association with thyroid autoimmune diseases, particularly Graves’ disease. Dysthyroid optic neuropathy is the most severe form of thyroid ophthalmopathy, and the optic nerve is compressed by the enlarged extraocular muscles, resulting in visual impairment. Dysthyroid optic neuropathy is relatively rare and accounts for less than 5% of thyroid ophthalmopathy cases in most studies. MRI can show optic nerve compression by swollen extraocular muscles, and it can demonstrate inflammation of extraocular muscles and orbital fat tissue with high-intensity signal on STIR images. The optic nerve in dysthyroid optic neuropathy is almost normal on conventional MRI. Attempts have been made to detect narrowing of the optic nerve diameter, using high-resolution T1-weighted images, but the results overlap with thyroid ophthalmopathy without visual impairment [50].