Introduction
Major aortopulmonary collateral arteries (MAPCAs) are a kind of rare cardiac congenital anomaly that involves embryologic junctions between the systemic and pulmonary circulation from the aorta or one of its branches to the pulmonary artery vasculature [1].
Some embryological studies indicate that the major aortopulmonary collateral arteries are persistent segmental arteries, not bronchial arteries. Early in foetal growth, the segmental arteries originating from the dorsal aortae are connected to the vascular plexus developing in the lung lobes. The vascular plexus within the lung is differentiated by the 40th day into separate segmental arteries and their branches, and the lung is supplied with blood by both the right ventricle and sixth branchial arches and segmental arteries up to around 50 days after ovulation. Some bronchopulmonary segments or lobules remain connected to the right ventricle, while others are related to the aorta. As the lung develops in a healthy foetus, it is exclusively supplied by central pulmonary arteries that originate from the sixth branchial arches. However, it appears that the normal maturation process fails in pulmonary atresia with ventricular septal defect [2]. However, the pathogenesis of MAPCA is not completely discovered.
Tetralogy of Fallot with pulmonary atresia and ventricular septal defect are the most common heart congenital diseases where MAPCAs emerge to compromise blood flow to pulmonary circulation. Around 10 per 100,000 live newborns experience pulmonary atresia (PA) with ventricular septal defect (VSD) and MAPCAs, a complex congenital cardiac disease [3]. Different lung segments receive varying blood flow from the collaterals due to their anatomical variability. The descending thoracic aorta is where the majority of the main aortopulmonary collateral arteries are situated. However, they may also be seen coming from the aortic arch, subclavian artery, distal thoracic aorta, internal mammary artery, and left coronary artery [3]. There are 3 types of MAPCA depending on the origin of the anomalous vessel. Type I describes MAPCAs coming from bronchial arteries. Type II covers branches arising directly from the aorta, and the last type is defined as indirect collaterals from the aorta via its branches, like the subclavian artery or celiac trunk [1]. Large AP collateral arteries showed reactivity and are susceptible to stenosis with time, which is histologically comparable to systemic arteries. It has been demonstrated that at least one region of stenosis develops in around 60% of MAPCAs [3]. Surgical management of patients with TOF, PA, and MAPCAs continues to be one of the most difficult tasks. Most patients die young if untreated, either from congestive heart failure and pulmonary vascular disease that progresses or from severe cyanosis and associated consequences. The overall mortality rate of patients who underwent single-stage complete unifocalisation (with or without final repair) varied from 10.2% to 19.2% [4]. This study aims to find correlations between congenital heart disease, clinical data, and the frequency and quantity of MAPCAs among patients admitted to our clinic between 2016 and 2023. Moreover, it also focuses on the origin of these collaterals in the CCTA series (Figures 1-4).
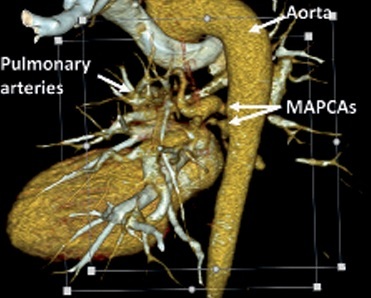
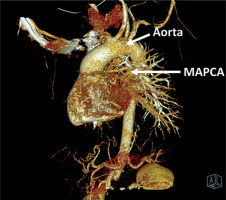
Figure 1
VRT (volume rendering technique) reconstruction of major aortopulmonary collateral arteries (MAPCAs). Computed tomography scan highlighting the aorta, pulmonary arteries, and MAPCAs and their anatomical relationships and blood flow pathways
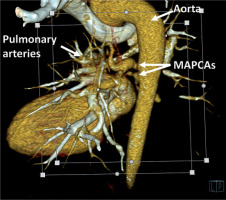
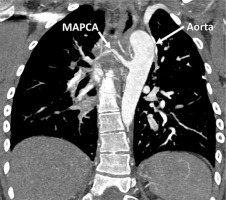
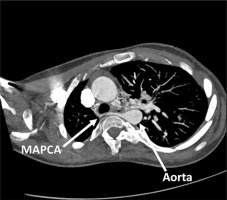
Figure 2
VRT (Volume Rendering Technique) reconstruction of major aortopulmonary collateral arteries (MAPCAs). Computed tomography scan highlighting the aorta and MAPCAs and their anatomical relationships and blood flow pathways
Methods
We evaluated 13,924 chest computed tomography exams performed in our heart imaging department between 2016 and 2023 on a SOMATOM Definition Flash dualsource 128-slice CT scanner, Siemens Healthineers, Forchheim, Germany, and established a retrospective registry of patients with MAPCA. The study included 46 cases. Clinical data were collected from medical records. The radiologic characteristics were obtained from the results, and if necessary, the image was reassessed to add details that might not have been evaluated routinely.
Results
Clinical characteristics
In Table 1, the number and percentage of patients with various congenital heart diseases are shown. The age range of the studied population varied from a one day living to 78 years old with an average of 12 years old and a median of 2 years old. Men accounted for 52.17% of the patients.
Table 1
Clinical characteristics of patients with congenital heart disease
Based on the information provided, it can be observed that among the total number of patients who underwent CCTA imaging, a significant proportion have been diagnosed with congenital heart diseases. The types of heart diseases in our study population included the following: tetralogy of Fallot (TOF), pulmonary valve atresia (PVA), pulmonary atresia (PA), ventricular septal defect (VSD), atrial septal defect (ASD), patent ductus arteriosus (PDA), and d-transposition of the great arteries (dTGA). There were 3 cases with another diagnosis. One of them was a right ventricular failure without a documented heart congenital disease, the second with suspicion of Rendu-Osler-Weber’s syndrome, and the last a case of a persistent left superior vena cava. Specifically, the CCTA series data revealed that 45.65% of patients had been diagnosed with TOF, while 26.09% had PVA, and 76.09% had PA. Additionally, 84.78% of patients had been diagnosed with VSD, while 21.74% had ASD, and 19.57% had PDA. Finally, 13.04% of patients had been diagnosed with dTGA. NT-proBNP is an established marker of cardiac dysfunction. The median value of NT-proBNP in our group was 412.1 pg/ml with a range from 74 pg/ml to 14,653 pg/ml according to the documentation of 44 patients for whom these numbers were available. These values exceeded the normal range (> 125 pg/ml) in 80% of patients. Saturation is a critical indicator of oxygenation and plays a pivotal role in monitoring patients with congenital heart diseases. In 50% of cases in this study population, the oxygen saturation ranged between 80% and 90%.
Echocardiographic findings
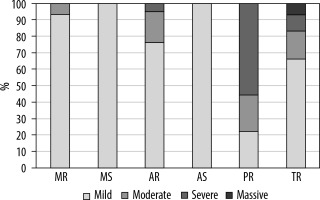
Echocardiographic data were available for 42 patients with MAPCAs. The most common finding was tricuspid regurgitation in 71.43% of patients, with 66.67% having mild, 16.67% moderate, 10% severe, and 6.67% massive regurgitation. The second most frequent finding (50%) was aortic insufficiency, with 76.19% being mild and 19.05% having moderate severity. Mitral regurgitation was present in 35.71% of patients, with the vast majority being mild (93.33%) and moderate (6.67%). The rarest findings were mitral stenosis (4.76%) and aortic stenosis (2.38%). The findings are summarised in Figure 5.
Radiologic findings
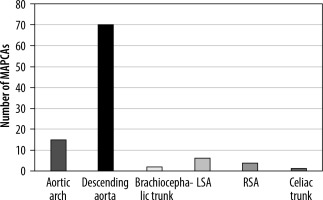
In our group of 46 patients, 100 MAPCAs were visualised. Patients had 1 to 5 of these arteries. Among 32.70% of patients, more than one MAPCA was present. Referring to Figure 6, we can see that type I MAPCA, which describes anomaly vessels arising from bronchial arteries [1], is rare and was not observed in our study population. Type II is the most common with a prevalence of 85%. The vast majority of this type consists of MAPCAs arising from the descending aorta (82.35%) in our group, and the rest were from the aortic arch (17.65%). Type III was observed in 13%, which contains MAPCAs arising from the brachiocephalic trunk, at 15.38%, from the left subclavian artery, at 46.15%, the right subclavian artery, at 30.77%, and the celiac trunk, at 7.69%
Figure 6
Origin of major aortopulmonary collateral arteries (MAPCAs). LSA – left subclavian artery, RSA – right subclavian artery
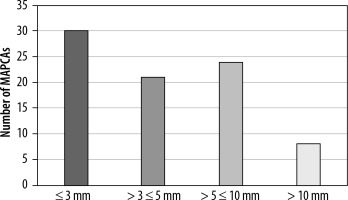
The data included 83 measurements of MAPCA diameter out of 100 available in the study. It was classified into 4 size ranges: ≤ 3 mm, > 3 mm and ≤ 5 mm, > 5 mm and ≤ 10 mm, and > 10 mm. The first group accounted for 36.14%, the second for 25.30%, the third for 28.92%, and the last one only for 9.64%. The median diameter of MAPCA was 4 mm. The largest was 16.5 mm, and the smallest was about 1 mm. The results are illustrated in Figure 7.
It appears that the most prevalent cases are those stemming from the descending aorta and typically involve very small vessels measuring less than 3 mm in diameter.
Discussion
The results demonstrate a correlation between congenital heart disease and the existence of MAPCAs. The highest number of cases were found with PA, TOF, and VSD as either an isolated lesion or a component of TOF. Numerous publications did not mention other congenital diseases like dTGA, which can also be associated with MAPCAs, as shown in our study. Sex did not correlate with the frequency of MAPCAs, and most cases it concerned young individuals. The valvular disorders mainly involving tricuspid and aortic regurgitation were often mild and therefore of low clinical significance.
Recent studies have focused on the issue of the coexistence of patent ductus arteriosus with MAPCA. One of these retrospective analyses examined all cases of foetuses over 10 years who had been diagnosed with MAPCA and had pulmonary atresia and an absence of PDA. In these patients, MAPCA was the only source of pulmonary blood flow [5]. Another study evaluating the characteristics and prognosis of 55 foetuses with pulmonary atresia and ventricular septal defect reported 19 cases with MAPCAs as the solitary blood supply to the lungs and 14 foetuses with a double blood supply (ductus arteriosus and MAPCAs) [6]. The ductus arteriosus and segmental arteries from the aorta persist to compensate for the pulmonary blood flow if the pulmonary arteries are not sufficiently developed. Both patent ductus arteriosus and MAPCAs may rarely coexist in the same patient [1]. In our study, 10 cases where varied numbers of MAPCA and PDA coexisted were found. If the patent ductus arteriosus, which develops from the underside of the aortic arch, distal to the subclavian origin, advances to join the proximal pulmonary artery without any intervening branches, the patient probably has collateral lung perfusion by MAPCAs [1,7]. It could be challenging to differentiate MAPCAs from a patent ductus arteriosus if they arise from the descending aorta at an elevated location. The absence of connection to the central pulmonary arteries at the bifurcation and the presence of several collateral vessels can be used as criteria for differentiating MAPCAs from patent ductus [7].
Moreover, the differentiating MAPCAs from bronchial arteries could be a diagnostic issue. Unlike bronchial arteries, MAPCAs do not have mediastinal branches, connect to larger pulmonary artery branches, and never form anastomoses with intercostal arteries [8].
A wide range of diameters was observed among the collaterals, and the exact values of each collateral are paramount for surgical intervention. In one analysis, vessels greater than 3 mm in diameter were considered major collaterals, while small collaterals were not clinically relevant and had little impact on the illness prognosis [1]. In another analysis, MAPCAs were referred to as big when they communicated directly with a pulmonary artery and were typically more than 2 mm in diameter. “Little” arteries were those connected indirectly [9]. However, in our study, all of the MAPCAs’ calibres were taken into account with proper significance, and the average diameter was about 5 mm, which has not been studied yet.
One recent study of 40 patients with PA-VSD in a multidetector computed tomography series showed that the number of MAPCAs in each case ranged from 1 to 5 collaterals, and the vessel sizes were irregular and ranged from 1 to 10 mm [10]. Our study also reported from 1 to 5 collaterals with the diameter ranging between 1 mm and 16.5 mm. In another paper, among 124 patients (with a total of 386 MAPCAs) with TOF or its variants with MAPCAs who underwent single-stage complete unifocalisation (with or without final repair) the origin of MAPCAs was examined, with most of them arising from the descending aorta (68.91%), followed by the subclavian artery (21%), aortic arch (8%), and ascending aorta (2%) [4]. This study confirmed our results, and the proportions were quite similar, indicating the descending aorta as the dominant origin of MAPCAs (Figures 1-4).
Conclusions
Our research highlighted the interaction between MAPCAs and congenital cardiac disorders.
Our findings revealed that MAPCA could exist without a diagnosed congenital heart failure. We also found connections with less common heart conditions, like dTGA, which suggests that conditions other than the most prevalent ones, such as TOF, PA, and VSD, could occur, which broadens our understanding of MAPCA pathogenesis.
Furthermore, the study emphasises the importance of monitoring biomarkers like NT-proBNP and oxygen saturation as valuable indicators of cardiac dysfunction in patients with MAPCAs.
Our radiologic results have shown the variety in MAPCA origin and size, highlighting the necessity for precise measurements in surgical planning. The prevalence of type II MAPCAs, which largely originate from the descending aorta, in our group, aligns with other studies (Figures 1-4).
The existence of MAPCAs greatly influences the prognosis of numerous congenital cardiac disorders involving pulmonary outflow anomalies [1]. Further prospective studies are needed to provide more information regarding the natural history of MAPCAs, their anatomy, and their impact on health.
To our knowledge, this is the first original paper presenting such detailed radiographic and clinical findings in this patient population.