Introduction
Medication-induced neurologic disorders are unintended adverse effects on the nervous system that are either caused by drugs or associated with drug use [1]. The same refers to biologic agents (hormones, interferons, interleukins, growth factors, monoclonal antibodies, polypeptides, proteins, vaccines), which differ from chemically derived drugs [2] but will be considered together in this review.
Most of the available literature focuses on adverse reactions of drugs from a specific group or used to treat specific diseases. Some publications present changes that occur due to administration of a certain drug in specific organs and systems of the human body and that can be visualized by imaging studies. It is much more difficult to find an overview of the possible drug-related causes of changes, starting from a particular organ. In this review the authors focus on abnormal brain magnetic resonance imaging (MRI) caused by drugs given to patients in any age group for any disease.
Viral infections with fever in children
“Aspirin is one of the most widely used medications in the world. Each year, 58 billion doses of aspirin are swallowed, sipped in fizzling concoctions or taken in suppositories, according to the Bayer Company, one of the largest manufacturers. Americans pop 80 million aspirin tablets daily – 29 billion per year – a figure that works out to 117 aspirin tablets annually for every man, woman and child” [3].
Reye syndrome is a rare acute noninflammatory encephalopathy with fatty liver failure. It usually starts with an infection of the upper respiratory tract followed by sudden onset of signs of raised intracranial pressure (vomiting, lethargy), leading to variable neurological deficits, including coma and death. On MRI, diffuse brain edema and T2-hyperintense thalami with restricted diffusion are reported [4].
A definite cause-effect relationship between aspirin intake and Reye syndrome in children has not been proven. No more than 0.1% of children who took aspirin developed Reye syndrome, but more than 80% of children diagnosed with Reye syndrome had taken aspirin in the preceding 3 weeks. These data led to recommendations against the use of aspirin in children in the 1980s. In 1986 the U.S. Food and Drug Administration (FDA) required that aspirin labels state that children and teenagers should not use the product, and since then, the number of reported cases of Reye syndrome has fallen dramatically [5].
The National Reye’s Syndrome Foundation, the U.S. Surgeon General, the FDA, the Centers for Disease Control and Prevention, and the American Academy of Pediatrics recommend that aspirin and combination products containing aspirin not be taken by anyone younger than 19 years during fever-causing illnesses. The British Medicines and Healthcare products Regulatory Agency recommends that aspirin labels state that aspirin is not for use in children younger than 16 years unless recommended by a physician [6].
Epilepsy
Corticosteroids have been used as antiepileptic drugs (AEDs) for decades and are known to impact the brain structure causing volume changes in both the white and gray matter of the brain. The loss of cerebral tissue may be a reversible effect of steroid administration, most likely due to the loss of water, and as such should not be reported as cerebral atrophy [7]. Having knowledge of the treatment of epilepsy with steroids, one will not attribute this appearance to a rare congenital disease, such as neuronal ceroid lipofuscinosis, the clinical manifestation of which is epilepsy, and on MRI brain atrophy may be the only finding [8].
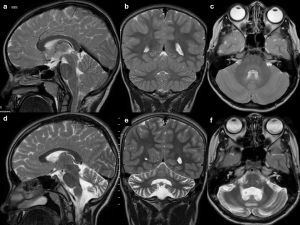
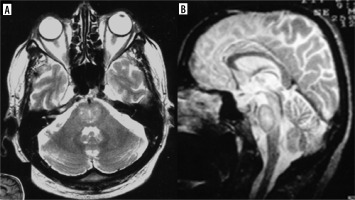
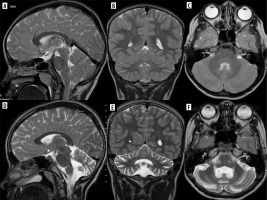
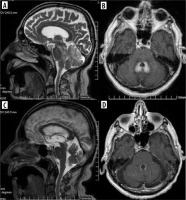
In contrast to the reversible loss of whole brain volume in patients treated with corticosteroids, another AED – phenytoin – causes cerebellar atrophy which may be irreversible, although in most cases cerebellar dysfunction regresses when phenytoin concentration is reduced (most patients with cerebellar signs have phenytoin concentrations above the reference range) [9] (Figure 1). Hyperostosis of the skull can also be observed in patients receiving phenytoin [10].
Figure 1
Drug-resistant epilepsy, responding only to high doses of phenytoin. T2-weighted images in three planes. Baseline study (top row, A-C), cerebellar atrophy on a follow-up study after 4 years (bottom row, D-F)
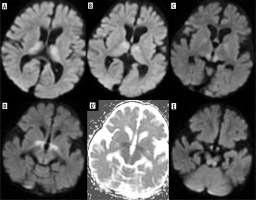
Vigabatrin is another AED known to cause brain abnormalities, most commonly occurring in the youngest patients, in the first year of life, especially with higher doses. Clinically, these abnormalities are usually asymptomatic. Brain MRI reveals symmetric T2 hyperintensities and diffusion restriction in the globi pallidi, thalami, dorsal brainstem, and dentate nuclei (Figure 2). These findings regress after vigabatrin withdrawal [11]. Without knowledge of this drug and its effects on the brain, one can consider MR images of the brain as similar to other rare diseases, such as Leigh syndrome, in which epilepsy can also occur [12].
Figure 2
An 11-month-old boy with epilepsy and trisomy 21 treated with vigabatrin. Consecutive DWI sections show a symmetrical pattern of thalamic, globi pallidi, anterior commissure, mamillary bodies and dorsal brain stem diffusion restriction, typical of vigabatrin side effects. D’ shows low apparent diffusion coefficient (ADC) values indicative of true diffusion restriction
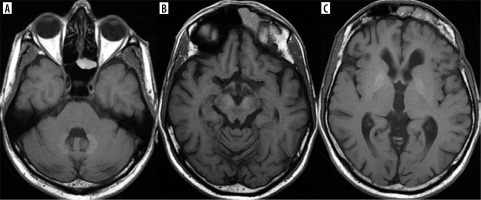
Some AEDs such as phenytoin, vigabatrin, and carbamazepine – when administered or suddenly withdrawn – may cause a reversible splenial lesion (RSL) that is currently more frequently called cytotoxic lesion of the corpus callosum (CLOCC). This T2 and FLAIR hyperintensity is located in the midline in the splenium of the corpus callosum, shows diffusion restriction and regresses with time [13] (Figure 3).
Psychiatric diseases
Cytotoxic lesion of the corpus callosum has also been reported in association with clinical neuroleptic malignant syndrome-like symptoms in patients treated with lithium due to schizophrenia or bipolar disorder [14].
Cerebrovascular effects have been attributed to antipsychotic agents. A significantly increased incidence of transient ischemic attack, cerebral ischemia, unspecified cerebrovascular disorders, and stroke has been reported in elderly patients with dementia-related psychosis. Individual stroke risk factors (smoking, hypertension, diabetes) increase the risk of this adverse neurologic event [15]. The MR appearance of stroke varies depending on how long it has been since the stroke occurred; the main advantage of MRI is that within minutes of arterial occlusion, it demonstrates diffusion restriction, i.e. an increased signal on DWI sequences with reduced apparent diffusion coefficient (ADC) values on the corresponding ADC maps.
Infections
Similar to vigabatrin and lithium, cerebellar dysfunction may be caused by metronidazole to treat bacterial or protozoal infections, especially during prolonged treatment, and CLOCC may be seen in these patients as well.
Metronidazole-induced lesions are in most cases symmetric in both cerebellar hemispheres, in the dentate nuclei. Additionally, they affect vestibular nuclei, tegmentum, and superior olivary nuclei. The lesions are T2- and FLAIR-hyperintense [11].
Patients infected with human immunodeficiency virus (HIV), especially type 1, may receive highly active antiretroviral therapy (HAART), which is a combination of several antiretroviral drugs. These patients may develop immune reconstitution inflammatory syndrome (IRIS), which is a paradoxical reaction to this treatment with deterioration of a pre-existing illness despite the positive influence of HAART on their immune function (increasing CD4+T-lymphocyte count and decreasing plasma HIV viral load). The MR appearances of the pre-existing lesions (caused by e.g. progressive multifocal leukoencephalopathy [PML], cryptococcal meningitis, tuberculosis) change in the case of IRIS, with marginal or perivascular contrast enhancement, marginal diffusion restriction and intrinsic gray matter T1 hyperintensity [16].
Multiple sclerosis
Immune reconstitution inflammatory syndrome may also develop in multiple sclerosis (MS) patients treated with natalizumab. Asymptomatic primary infection with John-Cunningham virus (JCV), which remains in latent form, is reactivated during the treatment due to immunosuppression, oligodendrocytes become infected and demyelination occurs. PML lesions must be differentiated from new MS plaques [17]. Diagnosis of PML results in natalizumab withdrawal, which in turn results in PML-IRIS with some distinct MR features described above.
Inflammatory diseases
Some of them (e.g. Crohn’s disease, ankylosing spondylitis, rheumatoid arthritis) are treated with tumor necrosis factor-α inhibitors (anti-TNFα) and this treatment – rarely – has the adverse action of promoting central nervous system (CNS) demyelination. In most cases it is optic neuritis and MS-like lesions, and – less frequently – aquaporin-4-IgG neuromyelitis optica spectrum disorder (NMOSD), and transverse myelitis, with well-known imaging findings [18].
Neoplastic conditions
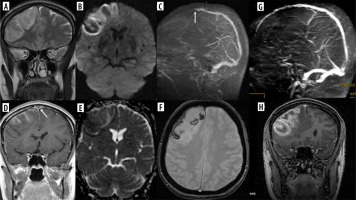
Immune checkpoint inhibitors (ICI) are monoclonal antibodies (with the name ending in “-umab” or “-imab”) used in numerous neoplastic diseases, such as melanoma, lung, renal cell, and breast cancer, glioblastoma, colon adenocarcinoma, and Hodgkin’s lymphoma. Similarly to anti-TNFα, they may cause CNS demyelinating events, including MS, NMOSD, optic neuritis, myelitis, PML (Figure 4) and atypical demyelination [18].
Figure 4
Progressive multifocal leukoencephalopathy (PML) in a 63-year-old woman with follicular lymphoma, treated with obinutuzumab (humanized type II glycoengineered, anti-CD20 monoclonal antibody) and bendamustine (alkylating agent). During treatment central paresis of the right facial nerve, then right hemiparesis, disturbances of consciousness, and – finally – loss of logical contact appeared. Multifocal, asymmetric lesions in both cerebral hemispheres with subcortical U-fiber involvement (FLAIR, A-D), no mass effect and contrast enhancement (G – before, H– after Gd administration); patchy diffusion restriction at the leading edge (DWI – E, corresponding ADC map – F, arrows)
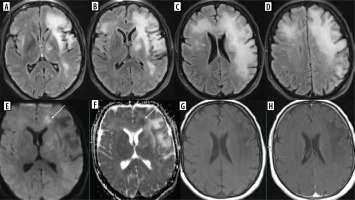
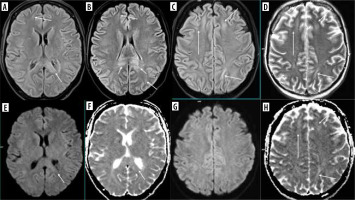
Methotrexate (MTX), as well as 5-fluorouracil, capecitabine and fludarabine, is known to cause transient brain injury that is reversable after discontinuation of treatment (Figure 5). This includes RSL as well. However, MTX-induced leukoencephalopathy may be diffuse and persistent [19]. The causative agent is difficult to establish if one drug that produces damage to the white matter is replaced by another that has similar side effects [20] (Figure 6).
Figure 5
DWI and corresponding ADC maps marked with apostrophes. Upper row: a 15-year-old boy with osteosarcoma of the fight femur treated with methotrexate. Brain MRI due to the first episode of seizures in his life. Trace of diffusion restriction in the right centrum semiovale (A, A’), otherwise normal brain. Middle row: second brain MRI 2 days later due to the worsening of the boy’s neurological condition. Clear bilateral diffusion restriction (C, C’ D, D’). Lower row: follow-up study 3 days after the previous one. Resolution of diffusion restriction after discontinuation of MTX – normal brain (E, E’, F, F’)
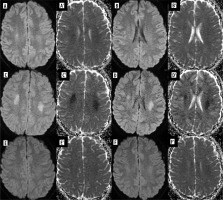
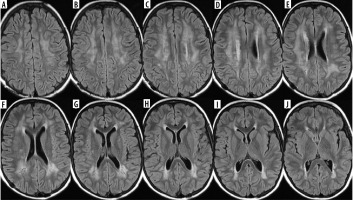
Figure 6
Consecutive FLAIR images (A-J) of the brain of a 12-year-old girl treated for osteosarcoma of the skull with methotrexate first and ifosfamide later on
It has to be mentioned here that intrathecal administration of MTX may result in spinal cord injury affecting the posterior columns with the same imaging appearance as subacute combined degeneration related to vitamin B12 deficiency, described as the inverted “V” sign or the “kangaroo ears” sign [21,22]. The same relates to intrathecal chemotherapy with cytarabine, cisplatin, carmustine, and thiotepa [22].
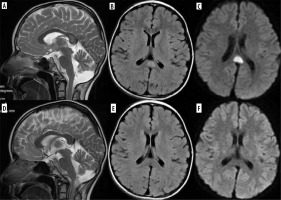
Both chemotherapeutics and monoclonal antibodies (e.g. bevacizumab) are associated with posterior reversible encephalopathy syndrome (PRES) presenting with acute neurologic symptoms, usually headache, visual disturbance, seizures, and altered mental state. Classic PRES presents as symmetric T2- and FLAIR-hyperintense posterior lesions without diffusion restriction, contrast enhancement, or deep nuclei involvement (Figure 7).
Figure 7
PRES in a 12-year-old girl treated for acute lymphoblastic leukemia (top row, A-C). Follow-up study 4 weeks later shows regression of lesions (bottom row, D-F)
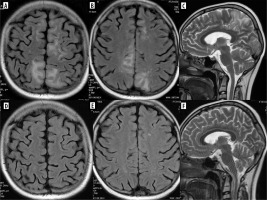
However, it may show these features and be also diffuse, unilateral, hemorrhagic [23]. PRES may also affect the spinal cord [24].
High-dose cytarabine and Minnelide (an experimental treatment for pancreatic and gastrointestinal cancers) are associated with REACT syndrome (reversible acute cerebellar toxicity), which is rare and potentially reversible, like PRES. The clinical symptoms include gait ataxia, dysmetria, imbalance, speech impairment, and acute confusion, and MRI reveals restricted diffusion in the cerebellar cortex with sparing of deep cerebellar nuclei and supratentorial brain [22].
Immunotherapy with CAR-T (chimeric antigen receptor T-cell) is used for the treatment of acute lymphoblastic leukemia and lymphomas and can result in remarkable clinical outcomes, but is associated with potentially fatal neurotoxicity: immune effector cell-associated neurotoxicity syndrome (ICANS). This is a clinical diagnosis with heterogeneous neuroimaging findings which can be grouped anatomically (involvement of the white matter, gray matter, brainstem, or leptomeninges) or pathologically (ischemic or hemorrhagic lesions, or cerebral edema) [25] (Figure 8).
Figure 8
Presumed ICANS in a 45-year-old woman with primary mediastinal lymphoma treated with CAR-T. After 3 weeks disturbance of consciousness, the patient was unconscious on the day of MRI. Discrete T2 (D) and FLAIR hyperintensities (A-C) in the frontal and parietal white matter, and in the corpus callosum (arrows). No diffusion restriction (DWI – E, G, corresponding ADC maps – F, H), no contrast enhancement. After about a month, the symptoms withdrew
Diseases requiring bone marrow or organ transplantations
PRES has also been described after the intake of immunosuppressants such as tacrolimus or cyclosporine.
Tacrolimus-induced brain injury may also present with mild chronic symptoms such as headache, and with diffuse symmetrical leukoencephalopathy involving both cerebral hemispheres and the brain stem [26] (Figure 9).
Figure 9
FLAIR. Tacrolimus-induced leukoencephalopathy in a 70-year-old woman 6 years after kidney transplantation. MRI performed due to dizziness and tremor. In this case the MR appearance could have been misdiagnosed as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy)
Dermatological diseases (especially acne vulgaris)
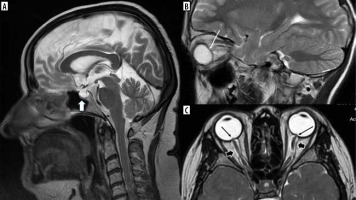
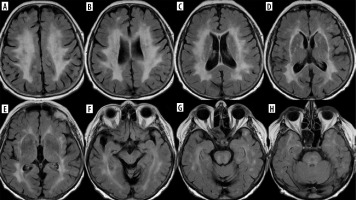
Vitamin A derivatives (especially isotretinoin) and tetracycline-class antibiotics are strongly associated with intracranial hypertension, which in these cases is not idiopathic but drug-induced (drug-induced intracranial hypertension – DIIH). As mentioned above, not only drugs used in dermatology may produce this effect: recombinant growth hormone and lithium were found to be most strongly associated with DIIH as well. Corticosteroids are moderately associated, and there is a long list of medications weakly associated with DIIH. The clinical picture of idiopathic intracranial hypertension (IIH), which is also known as pseudotumor cerebri syndrome, includes headache (predominantly frontal in location, worse in supine, and more prominent when first awakening), pulse synchronous tinnitus, and transient visual obscurations [27]. In IIH, there should be normal brain parenchyma without hydrocephalus, mass, or structural lesions and no abnormal meningeal contrast enhancement on MRI for typical patients (obese women), and on MRI and magnetic resonance venography (MRV) for other patients. However, there are signs of elevated intracranial pressure such as empty sella, flattening of the posterior aspect of the globe, distension of the perioptic subarachnoid space with or without a tortuous optic nerve, and transverse venous sinus stenosis. Transverse venous sinus asymmetry is present in many individuals (most have a dominant right transverse sinus), so it does not necessarily indicate pathology; however, bilateral venous sinus stenosis is rare in healthy individuals. [28]. Papilledema – if present – is also visible on MRI (Figure 10).
Diabetes
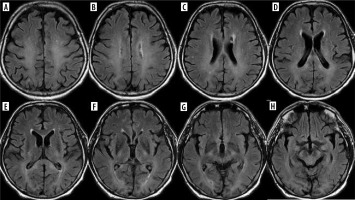
Insulin overdose may lead to cerebral injury due to hypoglycemia with rapid clinical deterioration from dizziness and palpitations to coma. The pattern of brain injury on MRI is bilateral symmetrical in most cases, similar to that seen in hypoxia, but with sparing of the thalamus, cerebellum, and brainstem. Diffusion restriction is found in the cortex of temporal and occipital lobes, of the insulae and hippocampi. The basal ganglia may be involved, and this is associated with a worse prognosis. Less commonly, T2 hyperintensity of the internal capsule, corona radiata, and splenium may also be seen, as well as isolated involvement of the white matter [29] (Figure 11).
Figure 11
Consecutive FLAIR images (A-H) of the brain of a 63-year-old hypoglycemic coma survivor. Symmetrical involvement of the white matter, including the corpus callosum (D, E)
Metformin causes metabolic encephalopathy in some patients with end-stage chronic kidney disease, with clinical symptoms of impaired consciousness and parkinsonism. A characteristic MR appearance of the lentiform nuclei has been described in these cases and named the “lentiform fork sign”. The name is derived from a bright, T2-hyperintense rim delineating the lateral and medial boundaries of the putamen. The medial arm splits into two branches on both sides of the globus pallidus (these branches represent the edematous medullary laminae dividing the lentiform nucleus into three parts: the putamen, globus pallidus interna and externa). The lateral arm of the fork represents the edematous external capsule. Together with a “stem” formed by the fusion of edematous external and internal capsules at the infero-posterior end of the putamen, they are meant to resemble a fork. Diffusion restriction is present in the globi pallidi [30].
Other disease entities and treatment modes
This part of this review will cover different disease entities, and the approach to CNS lesions will be presented from the perspective of drugs and treatment, rather than from the disease perspective, as in the previous part.
Total parenteral nutrition
Total parenteral nutrition is administered in various gastrointestinal tract diseases as well as in for example sepsis or polytrauma patients. It is typically enriched with minerals, including manganese (Mn), which in most patients leads to abnormally high serum Mn levels. Mn is deposited in the brain and may cause neurological symptoms in some patients, e.g. parkinsonism and psychiatric symptoms. Mn belongs to a small group of substances that cause shortening of T1 relaxation time and is therefore hyperintense on native T1-weighted images, similarly to other minerals, like calcium and copper, intra- and extracellular methemoglobin, lipids, high concentration of proteins, and melanin [31]. The most typical sites of Mn deposition are the globi pallidi, putamina, thalami, subthalamic nuclei, and pituitary gland [11,32] (Figure 12).
(Too) rapid correction of hyponatremia
Rapid correction of hyponatremia is the cause of myelin injury manifesting as central pontine myelinolysis (CPM), a component of osmotic demyelination syndrome (ODS). First attributed to alcoholism and malnutrition, CPM turned out to occur in other patients, in intensive care units in particular. Because these patients are not infrequently critically ill and have multiple metabolic derangements, it may be unclear when their neurologic impairment begins. An altered mental state is reported as the most common clinical symptom, followed by upper motor neuron pattern weakness [33]. The characteristic MRI finding in CPM is a T2-hyperintense lesion in the central part of the pons with an unaffected outer rim. Extrapontine lesions (extrapontine myelinolysis, EPM) may be found in various locations, such as caudate and lentiform nuclei, middle cerebellar peduncles, internal capsules, white matter and cortex of the cerebrum [34] (Figure 13). The earliest sign is diffusion restriction in the affected region.
Oral contraceptives
Oral contraceptives carry an increased risk of cerebral venous thrombosis (CVT) and are the most frequent gender-specific risk factor for CVT in women [35]. The term CVT includes dural venous thrombosis, cortical vein thrombosis and deep cerebral vein thrombosis. In about half of the cases CVT progresses to venous infarction. It is difficult to diagnose clinically and is not infrequently missed radiologically. MRI is the most sensitive and specific technique for diagnosis of CVT, and contrast-enhanced MR venography has the highest accuracy [36] (Figure 14).
Figure 14
A 44-year-old woman taking oral contraceptives, with a sudden, very severe headache, subsequent disorientation and finally seizures. Initial MRI study (A-F) shows hemorrhagic stroke of the right frontal lobe; the arrow points at an empty delta sign indicating thrombus in the superior sagittal sinus (SSS) on the post-contrast coronal scan (D) and at the SSS flow stop site on MR venography (C). Follow-up study 24 days later (G, H) shows recanalization of the SSS on MR venography (g; the whole SSS is visible) and lack of the empty delta sign (H)
Other medications with prothrombotic effects
There is a long list of drugs inducing thrombosis. It includes – among others – hormone replacement therapy (estrogens), antipsychotics, chemotherapeutic agents (e.g. tamoxifen), vascular endothelial growth factor (VEGF) antagonists, immunosuppressive agents (e.g. glucocorticoids, interferon-α, intravenous immunoglobulins), metformin, iodinated contrast media used on computed tomography (CT), and even – paradoxically – heparin, which may induce thrombosis via an auto-immune response to platelet-heparin complex [37].
Nitrous oxide (N2O)
The neurotoxicity of N2O relates to the spinal cord, but since such an effect of cancer chemotherapy has already been mentioned above, this drug is included here as well. Nitrous oxide, also known as laughing gas, is an inhaled anesthetic commonly used in dentistry. It interferes with cobalamin (vitamin B12) metabolism and presents on MRI in exactly the same way as classic vitamin B12 deficiency – with subacute combined degeneration of the spinal cord: T2-hyperintensity of the posterior columns [10].
Vaccines
Although no obvious causal relationship has been proven between vaccinations and encephalitis (in recent years mass vaccinations against SARS-CoV-2), cases of post-vaccination encephalitis have been reported, considering it an adverse effect of vaccines [38]. These reports included – among others – cases of acute disseminated encephalomyelitis (ADEM) (and acute hemorrhagic encephalitis, AHEM, both being a manifestation of myelin oligodendrocyte glycoprotein antibody-associated disease, MOGAD) which is well known to occur after vaccinations. The MRI characteristics includes bilateral, longitudinally extensive optic nerves involvement, with the optic nerve sheaths, longitudinally extensive lesions in the spinal cord including the conus, with characteristic grey matter involvement leading to the H-sign, fluffy or poorly demarcated T2-hyperintense lesions in the brain, involving white matter, deep grey matter, middle cerebellar peduncles, brainstem, and cortex, with non-specific leptomeningeal and/or cortical contrast enhancement, with partial or complete resolution [39]. As reported in numerous publications, it is suggested that reactivation of pre-existing neuroinfections may take place after vaccinations, as was likely the case in our patient with presumed tick-borne encephalitis (Figure 15).
Figure 15
Brainstem and cerebellar encephalitis in an 87-year-old man with nystagmus, trunk ataxia, dysarthria, marked balance disorders, abolished vibratory sensation in the lower limbs and trunk from the rib arches downwards, and the only potential cause being vaccination against COVID-19 4 weeks prior to the illness. T2-weighted sagittal images (A, C), T1-weighted axial images with gadolinium enhancement (B, D). Maximal changes 4 weeks after disease onset (A, B). Marked regression 2.5 weeks later (C, D)
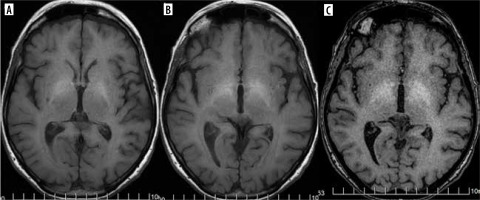
Gadolinium deposition in the brain
Gadolinium-based contrast agents are not prescribed medications but they constitute an indispensable element of many magnetic resonance (MRI) scans. They have been used for at least 36 years now. In 2014 Kanda et al. reported T1-hyperintense dentate nuclei and globi pallidi on unenhanced MRI and concluded that these might result from previous gadolinium-based contrast agents (GBCA) administrations [40] (Figure 16). Since then it has been a well-known fact that gadolinium deposition occurs with all contrast agents, although linear agents appear to deposit more than macrocyclic ones. No adverse biologic or clinical effects from gadolinium deposition have been reported to date [41]. However, in line with the ALARA principle (as low as reasonably achievable; this principle has so far been applied to ionizing radiation, ensuring that its dose to the patient is the lowest possible) and the recommendations of European and American regulatory agencies, it has been stated that GBCA administration should be minimized and only indicated if it is essential for the patients’ clinical management [42].
Conclusions
Medication-induced changes should be considered in the differential diagnosis of neurologic disorders with abnormal brain MR images.
The knowledge of the patients’ diseases and the medications they take is crucial to establish the correct diagnosis. The absence of these data in a referral for an MR brain scan can result in completely wrong suspicions and unleash unnecessary, complicated, time-consuming and expensive diagnostics causing additional stress in patients and their guardians.
Recreational drugs and other addictive substances are not the subject of this review because they are not prescribed medications, although knowledge of their use is equally essential for diagnosis.