Introduction
Impacted teeth are those that do not erupt into the dental arch within the expected developmental window and have no clinical or radiographic evidence of imminent eruption [1]. After the third molar, the permanent maxillary canine has the highest impaction rate, occurring in about 1-2% of the human population [2]. According to Archer, maxillary canine impaction occurs in 5 forms of buccal, palatal, buccopalatally oblique, in-alveolar, and inverted [3]. The incidence of palatal impaction, with a prevalence of 0.8-3%, is 3-6 times more frequent than the buccal type [4]. Unlike third molar impaction, this impaction is important in aesthetics and function. Left untreated, it can cause tooth loosening, root resorption, dentigerous cyst formation, and anomalies such as tooth agenesis, transposition, crowding, and crossbite along the jaw arch [5-8].
Determining the position of the impacted tooth and its effects on the adjacent structures, such as the thickness and height of the alveolar bone, the width and perimeter of the maxillary arch, and the angle of the maxillary incisor roots, are critical variables in diagnosis and orthodontic treatment of these cases [9]. Radiographic images can be used to assess these factors; however, conventional modalities are not recommended due to the superimposition of the surrounding structures on the film.
Nowadays, cone-beam computed tomography (CBCT) images have surpassed the conventional methods by providing more accurate and reliable findings [10]. CBCT radiography accurately identifies the position of dental structures, anomalies, and impacted teeth. These images have no magnification error and can measure the exact linear and angular examinations. Detecting the location of the dental follicle, the amount of bone covering the crown and root analysis of the adjacent tooth, and assessing bone changes affected by the impacted tooth are some of the other benefits of CBCT [11].
The relationship between canine impaction and the morphological features of the maxillary bone has recently captured researchers’ attention [12-18]. Some studies have reported a relationship between the skeletal and dental width of the maxilla and canine palatal impaction [9,14,19]. Several investigations have found that the posterior crossbite or reduction of the transverse dimension of the maxilla is related to canine impaction. Also, some studies claim that space problems and eruption problems could lead to maxillary canine impaction. Still, they do not clarify the impact of the buccal or palatal impaction side. Some claim that patients with canine palatal impaction have a larger maxilla in the transverse dimension, while others do not find a significant relationship. Moreover, several researchers state that disturbance in the transverse dimension of the maxilla increases the possibility of canine impaction; however, others have observed no significant difference between the anteroposterior width of the maxillary arch and canine impaction. Several studies have suggested that patients with palatal-embedded canine teeth have larger transverse dimensions of the maxillary bone [14-16,20].
Considering the controversies above and the few available studies evaluating the relationship between canine impaction and the dimensions of the maxillary bone in the Iranian population, this study intended to investigate the association between the position of maxillary unilaterally impacted canines and the morphological features of the maxilla in an Iranian population based on CBCT images.
Material and methods
This retrospective split-mouth study was in accordance with the Helsinki Declaration and was approved by the Research Ethics Committee of Mazandaran University of Medical Sciences (code: IR.MAZUMS.REC.1400.473). This study assessed the maxillary impacted canine teeth on the CBCT images of patients referred to an oral and maxillofacial radiology centre from orthodontic offices in Sari, Iran, from 2018 to 2021. All the patients whose images were included in the study were thoroughly informed of the purpose and procedures of the study, and written informed consent was obtained from each of them prior to the study. An unerupted canine was considered impacted when its root development was complete or the contralateral canine had wholly erupted. The full eruption was identified as the tooth being in its expected position and occlusion based on a radiograph [21]. Finally, palatal and buccal impactions were specified as palatal and buccal placement of the canine incisal edge compared to the apex of the adjacent lateral incisor [22].
The sample size of 94 patients with unilaterally impacted maxillary canine teeth (47 cases of buccal impaction and 47 cases of palatal impaction) was estimated based on the study of Shahin et al. [23], considering the α = 0.05, power of 80%, μ1 = 35.5, μ2 = 33.2, sd1 = 4.27, and sd2 = 3.67 as follows:
Patients with high-quality CBCTs and an appropriate field of view and no missing or extracted teeth were included. On the other hand, cases with a previous history of orthodontic treatment, craniofacial anomalies, maxillary dental implants, maxillary surgery, and systemic conditions were excluded from this study.
According to the inclusion and exclusion criteria, CBCT images of 164 patients with an impacted maxillary canine were assessed, and 94 were finally considered. In addition, all patients had panoramic radiography from the same centre. All CBCT images were taken via a Carestream CS 9300 CBCT scanner (90 kV, 10 mA, 11 × 11 cm2 field of view, 100 µm voxel size, Carestream Dental LLC, Atlanta, USA). Two experienced orthodontists simultaneously assessed the images and measurements. The buccal or palatal position of the impacted canine teeth was determined based on the CBCT images.
Based on the radiographic findings, the 7 parameters of alveolar height, alveolar thickness, nasal width, maxillary arch width (in canines), maxillary arch perimeter, palatal depth, and palatal volume were measured for comparison between the impacted/non-impacted side and also between the buccal/palatal groups. These parameters were measured as follows [21,22]:
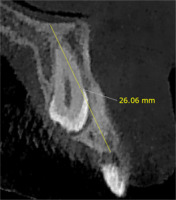
The alveolar bone height around the canine was measured from the level of the crest (Reference 1) to the floor of the nasal fossa, the height on the impacted side was measured along the longitudinal axis of an imaginary endosseous implant, and the height on the non-impacted side was measured along the longitudinal axis of the canine (Figure 1).
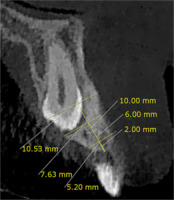
The alveolar bone thickness around the canine was measured in the sagittal plane at 3 depths of 2, 6, and 10 mm apically to the alveolar crest. The buccopalatal thickness was measured from the centre of the edentulous part on the impacted side and the centre of the canine on the non-impacted side (Figure 2).
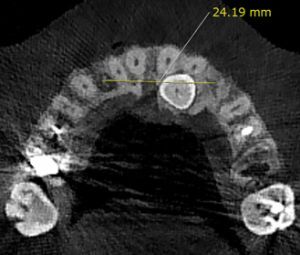
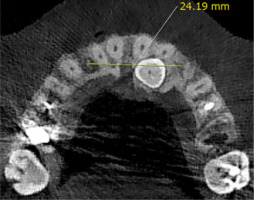
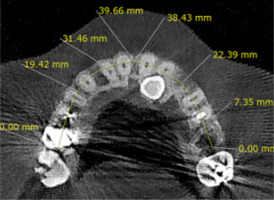
The maxillary arch width on the impacted side; the distance from the median palatine raphe to the interproximal bone between the impacted canine tooth and the maxillary first premolar was measured at the centre of the buccopalatal thickness of the bone. On the non-impacted side, the distance between the median palatine raphe and the interproximal bone of the erupted canine tooth and the first premolar was measured. This parameter was measured in the axial section at the crestal bone surface level (Figure 3).
The maxillary arch perimeter was measured from the distal of the first molar to the intermaxillary suture on both impacted and non-impacted sides at the cervical third of the teeth (Figure 4).
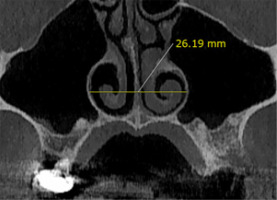
The nasal width. The length of the line connecting the widest segment in the lower third of the nasal cavity on one side to the opposite side in the former section was measured. In addition, the width of each side of the nasal cavity was measured, considering the centre of the nasal spine (Figure 5).
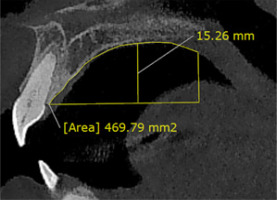
The palatal volume and depth. The palate volume was measured in mid-sagittal view. The area of interest included the upper, lower, anterior, and posterior vectors, referred to as: (1) upper vector – in the mid-sagittal view, the highest point of the palatal vault; (2) lower vector – a line drawn at the cementoenamel junction (CEJ) of the central incisor parallel to the horizon; (3) anterior vector – connecting line of CEJ central incisor to the upper vector; and (4) posterior vector – the line from the posterior nasal spine (PNS) perpendicular to the lower vector (Figure 6). The 3D palate model was created in Mimics software version 21 using the dynamic region growing tool. Thus, the volume of the palate was provided using this software. A line from the upper vector, perpendicular to the lower vector, was calculated as the depth or height of the palate (Figure 6).
Data were analysed using descriptive statistics, including mean ± standard deviation. Frequency tables were used for qualitative data such as gender. A statistical t-test was used to compare the results between the buccal and palatal impaction groups. Data were analysed using SPSS (version 16; Chicago, Ill) with a significance level of 0.05.
Results
The study participants were 94 patients, 29 (30.9%) males and 65 (69.1%) females, with a mean age of 19 years, ranging from 15 to 27 years. According to Table 1, there was no significant association between gender and age with the type of impaction.
Table 1
The relationship between demographic variables and type of impaction
| Demographic variable | Buccal impaction group | Palatal impaction group | p-value | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 19 (65.5) | 46 (70.8) | 0.739* | |
| Male | 10 (34.5) | 19 (29.2) | ||
| Age, mean (SD) | 19.80 (3.19) | 18.50 (2.55) | 0.152** | |
Palatally impacted canines
As Table 2 discloses, based on the findings in the palatal impaction group, the alveolar bone height was not significantly different between the impacted and non-impacted sides (p = 0.840). The alveolar bone thickness at a depth of 2 mm was significantly lower on the impacted side than the non-impacted side (p = 0.012). However, this factor was not significantly different at the depths of 6 and 10 mm (p = 0.273 and p = 0.443, respectively). The maxillary arch width was significantly greater on the non-impacted side (p = 0.007). No significant difference was observed in terms of the maxillary arch perimeter between the 2 sides (p = 0.202). In addition, Table 2 reveals no significant difference regarding nasal width between the impacted and non-impacted side (p = 0.307). However, the palatal volume was significantly greater on the non-impacted side (p = 0.031).
Table 2
Comparing the parameters between the impacted and non-impacted sides
| Variable | Side | Palatal impaction group (n = 47) | Buccal impaction group (n = 47) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | t-paired | p-paired | Mean ± SD | t-paired | p-paired | ||
| Alveolar bone height | Impacted | 18.45 ± 2.16 | 0.20 | 0.840 | 20.24 ± 2.46 | 1.99 | 0.061 |
| Non-impacted | 18.55 ± 3.21 | 19.16 ± 3.07 | |||||
| Alveolar bone thickness at a depth of 2 mm | Impacted | 7.51 ± 1.92 | 2.78 | 0.012 | 7.75 ± 1.60 | 2.71 | 0.014 |
| Non-impacted | 8.64 ± 1.29 | 8.95 ± 1.17 | |||||
| Alveolar bone thickness at a depth of 6 mm | Impacted | 8.25 ± 1.55 | 1.13 | 0.273 | 9.28 ± 1.50 | 0.87 | 0.395 |
| Non-impacted | 8.65 ± 1.82 | 9.03 ± 1.30 | |||||
| Alveolar bone thickness at a depth of 10 mm | Impacted | 9.24 ± 1.75 | 0.78 | 0.444 | 10.29 ± 1.59 | 2.44 | 0.024 |
| Non-impacted | 9.05 ± 2.00 | 9.62 ± 1.69 | |||||
| Maxillary arch width | Impacted | 14.24 ± 2.12 | 2.99 | 0.007 | 15.17 ± 1.84 | 3.62 | 0.002 |
| Non-impacted | 15.47 ± 1.57 | 16.02 ± 1.39 | |||||
| Maxillary arch perimeter | Impacted | 30.90 ± 2.42 | 1.32 | 0.202 | 30.18 ± 3.11 | 2.93 | 0.008 |
| Non-impacted | 31.61 ± 2.52 | 31.65 ± 3.02 | |||||
| Nasal width | Impacted | 29.87 ± 2.27 | –1.04 | 0.307 | 30.27 ± 2.37 | –1.01 | 0.307 |
| Non-impacted | 30.58 ± 2.16 | 31.01 ± 2.39 | |||||
| Palatal volume* | Impacted | 4449 ± 1128 | –2.15 | 0.031 | 4503 ± 1631 | –2.33 | 0.020 |
| Non-impacted | 4933 ± 1166 | 5103 ± 1339 | |||||
Buccally impacted canines
Table 2 shows no significant difference in the alveolar bone height on both impacted and non-impacted sides in the buccal impaction group (p = 0.061). The alveolar bone was significantly thinner on the impacted side at a depth of 2 mm (p = 0.014). However, no significant difference was observed between the 2 sides at a depth of 6 mm (p = 0.395). This variable was significantly higher on the impacted side at a depth of 10 mm (p = 0.024). The maxillary arch width and perimeter were significantly greater on the non-impacted side (p = 0.002 and p = 0.008, respectively). Moreover, Table 2 shows no significant difference between the impacted and non-impacted sides regarding nasal cavity width, but a significant difference was observed in palatal volume (p = 0.307 and p = 0.02, respectively).
Non-impacted and impacted sides
As Table 3 illustrates, no significant difference was found in any of the variables when comparing the non-impacted sides in the buccal and palatal impaction groups. Furthermore, Table 3 compares the impacted side between the buccal and palatal groups and indicates that the alveolar bone height was significantly higher in the buccal group compared to the palatal group (p = 0.016). Nevertheless, no significant difference was observed concerning the alveolar bone thickness at a depth of 2 mm (p = 0.661). However, this parameter was significantly higher in the buccal impaction group at a depth of 6 and 10 mm (p = 0.036 and p = 0.049, respectively). In addition, no significant difference was noticed between the groups regarding maxillary arch width and perimeter, palatal volume and depth, and nasal width (p > 0.05).
Table 3
Comparing the parameters between the palatal and buccal groups
| Variable | Group | The non-impacted side (n = 47) | The impacted side (n = 47) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | t | p | Mean ± SD | t | p | ||
| Alveolar bone height | Palatal | 18.55 ± 3.21 | –0.628 | 0.534 | 18.45 ± 2.16 | –2.513 | 0.016 |
| Buccal | 19.16 ± 3.07 | 20.24 ± 2.46 | |||||
| Alveolar bone thickness at a depth of 2 mm | Palatal | 8.64 ± 1.29 | 0.818 | 0.418 | 7.51 ± 1.92 | –0.444 | 0.661 |
| Buccal | 8.95 ± 1.17 | 7.75 ± 1.60 | |||||
| Alveolar bone thickness at a depth of 6 mm | Palatal | 8.65 ± 1.82 | –0.780 | 0.440 | 8.25 ± 1.55 | –2.174 | 0.036 |
| Buccal | 9.03 ± 1.30 | 9.28 ± 1.50 | |||||
| Alveolar bone thickness at a depth of 10 mm | Palatal | 9.05 ± 2.00 | –0.998 | 0.324 | 9.24 ± 1.75 | –2.027 | 0.049 |
| Buccal | 9.62 ± 1.69 | 10.29 ± 1.59 | |||||
| Maxillary arch width | Palatal | 15.47 ± 1.57 | –1.220 | 0.230 | 14.24 ± 2.12 | –1.515 | 0.138 |
| Buccal | 16.02 ± 1.39 | 15.17 ± 1.84 | |||||
| Maxillary arch perimeter | Palatal | 31.61 ± 2.52 | –0.047 | 0.696 | 30.90 ± 2.42 | 0.853 | 0.408 |
| Buccal | 31.65 ± 3.02 | 30.18 ± 3.11 | |||||
| Nasal width | Palatal | 30.58 ± 2.16 | –0.605 | 0.552 | 29.87 ± 2.27 | –0.554 | 0.585 |
| Buccal | 31.01 ± 2.39 | 30.27 ± 2.37 | |||||
| Palatal volume* | Palatal | 4933 ± 1166 | –0.504 | 0.614 | 4449 ± 1128 | –0.365 | 0.715 |
| Buccal | 5103 ± 1339 | 4503 ± 1613 | |||||
| Palatal depth | Palatal | 15.26 ± 1.76 | –0.514 | 0.613 | 14.90 ± 1.76 | –0.454 | 0.655 |
| Buccal | 15.54 ± 1.81 | 15.15 ± 1.85 | |||||
Discussion
This analytical-descriptive cross-sectional study was conducted on samples with unilateral maxillary canine impaction. All samples were examined separately regarding alveolar bone thickness and height and maxillary arch width and perimeter. Previous studies had either measured fewer parameters or only examined palatal impaction [19,24-26]. Thus, this study aimed to conduct a more comprehensive measurement by considering more variables and simultaneously examining both buccal and palatal impaction.
The 94 patients were divided equally into palatal and buccal impaction groups. Patients had a mean age of 19 years. The findings represented no significant relationship between gender or age and the canine impaction type. Moreover, no significant difference was observed in the height of the alveolar bone on both impacted and non-impacted sides in the palatal and buccal impaction groups. Consistent with the present study, D’Oleo-Aracena et al. [27] found similar results on the palatal side. Conversely, Tadinada et al. [21] revealed that the height of the alveolar bone on the non-impacted side was significantly higher than the impacted side and justified this difference with the presence of teeth. The difference in these studies might be due to differences in sample size or measurement errors.
The present study suggested a significantly thinner alveolar bone on the impacted side at a depth of 2 mm. However, this difference was not significant at a depth of 6 and 10 mm. Similarly, Tadinada et al. [21] demonstrated a significantly thinner alveolar bone on the impacted side at a depth of 2 mm and no significant difference in the depth of 6 mm; nevertheless, unlike our study, the thickness was significantly lower on the impacted side compared to the non-impacted side at a depth of 10 mm. It should be noted that the alveolar ridge resorption happens in a specific location in the absence of a specific tooth (extraction or impaction), and compared to vertical bone reduction, the amount of bone resorption is greater in the horizontal direction, which affects the thickness of the bone [28,29]. Thus, greater thickness on the impacted side in the present study can be explained by the possibility that the impacted teeth are closer to the ridge. Due to the possible presence of a hidden tooth at a depth of 6 or 10 mm, it can be justified that the alveolar bone thickness at this height is similar on both impacted and non-impacted sides.
This study revealed that the alveolar bone thickness at a depth of 2 mm was significantly lower on the impacted side compared to the non-impacted area in the buccal impaction group. However, this parameter was not significantly different at a depth of 6 mm. These findings are consistent with those found in the palatal group and can be explained by the same reasons. Additionally, the alveolar bone was significantly thicker on the impacted side than the non-impacted side in the buccal group at a depth of 10 mm.
The maxillary arch width was found to be significantly lower on the impaction side in both the buccal and palatal groups. Unlike our findings, Al-Nimri et al. [24] and Tadinada et al. [21] reported a greater maxillary width on the impacted side. However, Mohammed et al. [9] measured the width of the palatal arch in patients with normal canine eruption, unilateral canine impaction, and bilateral canine impaction using a different method and found no significant difference. Moreover, other investigations suggested different results [19,20,25,27]. These discrepancies may be due to the differences in ethnicities investigated or the methodology used in the studies.
This study demonstrated no significant difference in the palatal impaction group regarding maxillary arch perimeter (p = 0.20). Likewise, Jacoby et al. [30] discovered that 85% of palatal impactions occur in patients with an appropriate arch perimeter. In addition, Stellzig et al. [31] showed sufficient arch space in 82% of patients with palatal impaction. Therefore, it is noteworthy that no reduction in the arch perimeter is necessarily observed on the side with palatally impacted canines.
In the buccal group, the maxillary arch perimeter was significantly smaller on the impacted side. Similarly, Kim et al. [22] illustrated that the shape of the maxillary arch in people with unilateral canine palatal impaction is narrower, and the intermolar width is less in comparison to buccal impaction; these differences can justify more space in the impacted side in buccal impaction. According to their results, palatal impaction occurs in deep palatal vaults, and the direction of these impacted teeth is usually horizontal or semi-horizontal, which does not affect the arch environment. Due to the low thickness of the buccal bone, buccal impaction requires occupying space and an apparent protrusion in the buccal, which naturally increases the arch perimeter.
There was no significant difference between the impacted and non-impacted sides in none of the buccal or palatal groups of this study. Also, no significant difference was found between the palatal and buccal groups in any of the impacted and non-impacted sides. In agreement with our study, Saiar et al. [19] and Miresmaeili et al. [32] found no relationship between the nasal width and the impaction of the canine in the upper jaw.
In this study, we have found that patients had a significantly greater palatal volume on the normal sides of the maxilla than the impacted side in both groups of buccal and palatal impaction. However, the comparisons showed no significant difference between the palatal and buccal groups in any of the impacted and non-impacted sides in regard to the palatal volume. Consistent with this study, Yassaei et al. [33] found that the patients had a significantly greater palatal volume on the non-impacted side than on the impacted side.
The results of this study showed no significant difference between the palatal depth of buccal and palatal impaction groups in any of the impacted and non-impacted sides. In the study of Fattahi et al. [34] there was no significant difference between the palatal and buccal canine impaction and control groups, which was in agreement with our study. However, Salim et al. [35] reported a significant difference among control, unilateral, and bilateral impaction groups. Contrary to the results of this study, Shahin et al. [23] also reported that the patients without impacted canines had a significantly deeper palatal vault than the patients with impacted canines.
Other novel findings of this investigation included comparing four variables of thickness and height of the alveolar bone and width and perimeter of the maxillary arch on the buccal impaction group in comparison with the palatal group, which revealed no significant difference. Nevertheless, in comparing the same parameters between the non-impacted side in the buccal and palatal groups, the alveolar bone height and thickness were significantly higher in the buccal group at a depth of 6 and 10 mm.
The findings of this study suggest that the morphological features of the maxillary arch, especially the maxillary arch width, palatal volume, and the alveolar bone thickness, might be used as a risk indicator for the diagnosis of buccal or palatal maxillary canine impaction. When necessary, diagnosis through clinical examination and conventional imaging can be supplemented by CBCT radiography [36]. Analysing how the abovementioned features differ from normal maxillary morphology can help the orthodontists to detect the canine eruptive problems in the early stages, explain the situation to patients, and plan the most appropriate treatment options [37].
Similarly to other studies, this study is subject to some limitations. Despite the examination of CBCT images by a radiologist, measurement errors are always possible. Therefore, contradictions in these findings require further studies with greater sample sizes. Furthermore, because most studies evaluated only palatal impaction of maxillary canines, it is suggested that more studies be performed investigating both palatal and buccal impaction to obtain more accurate results. In addition, it is suggested that additional variables be measured in future investigations, which influence impaction severity and treatment outcomes, such as root angulation and proximity to adjacent teeth.
Conclusions
The most significant findings of the present study in an Iranian population show that reduced palatal volume, maxillary arch width, and maxillary arch perimeter could be considered as contributing factors to the maxillary unilateral canine impaction. In addition, the findings regarding alveolar bone thickness showed contrary results at different depths; it was significantly lower on the impacted side in both palatal and buccal impaction groups at a depth of 2 mm, and significantly higher on the impacted side in the buccal group at a depth of 10 mm. Moreover, the comparisons showed that the maxillary alveolar bone had significantly greater height around the buccally impacted canines than the palatally impacted ones.