Introduction
A normal pulmonary venous pattern with 4 separate veins and differentiated ostia may be seen in up to 60-70% of the population [1]. Failure of normal embryological development of pulmonary veins may lead to a spectrum of congenital anomalies, ranging from normal variation to partial anomalous pulmonary venous connection (PAPVC) and complete anomalous pulmonary venous connection (TAPVC). Such anomalous pulmonary venous variations may be seen in up to 38% of the population [2]. The ano-maly may be isolated with the presence of an associated atrial septal defect (ASD), or it may present as an association of complex congenital cardiac disease (CHD). Morphological heterogeneity and associated congenital anomalies act as major determinants of morbidity and mortality in PAPVC [3,4]. Therefore, accurate and early diagnosis of the condition and associated anomalies is crucial for decision-making and definitive management. Nowadays multidetector computed tomography angiography (MDCTA) and MR angiograms (MRA) have nearly replaced the traditionally used imaging techniques, helping considerably in optimal assessment. This article gives comprehensive details of PAPVC and its variations using MDCTA imaging.
Relevant embryological development of pulmonary veins
Different theories have been put forward regarding the development of pulmonary veins on the basis of animal and human studies [5]. As per the most accepted theory, primitive lung buds originate from the foregut and receive a systemic arterial supply and drain into the cardinal venous system [6,7]. A small outpouching from the left atrium develops into a primitive common pulmonary vein (CPV), which communicates with the splanchnic plexuses and establishes a connection with the pulmonary venous system. With further development, the venous connection with the cardinal system disappears and the CPV becomes assimilated into the dorsal portion of the left atrium and finally differentiates into 4 separate pulmonary veins to drain the blood from the lungs [8].
Aberration from the normal development may result in anomalous pulmonary venous anomaly. The persistence of communication with the splanchnic plexus, cardinal, and umbilicovitelline vein, and failure of connection of the CPV with the splanchnic plexus, which leads to heterogeneity in anomalous pulmonary venous connections.
Normal anatomy and variations
Four pulmonary veins (PV) with separate venous ostia draining into the left atrium (LA) form a normal pulmonary venous anatomy in most of the population. The right upper and lower PVs drain the respective lung lobes with additional drainage of the middle lobe by the upper pulmonary vein. Similarly, the left upper and lower PVs drain the left upper and lower lobes, respectively, and the lingula is additionally drained by the left upper PV.
Conjoined pulmonary veins and/or accessory pulmonary veins have been reported as the commonest PV variations [9]. A common venous channel formed by the right or left upper and lower PVs, which connects to the left atrium by a single ostia, is termed as conjoined vein. The accessory veins are the veins additional to the normal upper or lower PVs, which drain separately into RA and usually have a smaller ostium [10,11]. Reports say that right-sided variations, though less common, are more complex. These can present as one or two accessory right middle PVs, or one accessory right middle PV and one accessory right upper PV [12].
Role of imaging
Traditionally, X-ray of the chest and echocardiography are the initial investigations in a patient with suspected CHD. The radiograph helps in assessing the cardiac size and contour, giving a gross projectional anatomy of the heart chambers. The status of the lungs, central pulmonary vessels, and interstitial pulmonary oedema can be also evaluated on a frontal projection; however, assessment of a root cause for such changes remains uncertain. Echocardiography is an excellent tool for evaluation of cardiac anatomy and function. It remains the initial investigation of choice for the assessment of CHD including anomalous pulmonary venous connections [13,14]. Transthoracic and transoesophageal echocardiography (TEE) are both used, but the latter is preferred by many clinicians due to its better sensitivity and efficacy. However, TEE usually requires general anaesthesia because most of the affected patients are young children [15,16]. Also, assessment of extra-cardiac pathology is usually suboptimal on TEE; therefore, its findings need to be confirmed with a better alternative investigation (like CT/MR angiography).
Cardiac catheterization has frequently been used in the past for detailed evaluation of CHD including pulmonary venous connections, but its use has declined in the last decade with the advent of newer imaging tools like MDCT and MRI. Being an invasive procedure, it requires conscious anaesthesia and has its related risks, which far greater more than noninvasive techniques. The procedure is lengthy, with more radiation exposure and hence related risks. Cardiac catheterization also lags behind newer techniques in providing 3-dimensional imaging.
There has been definite technical advancement in computed tomography in the last decade. The newer MDCT with high spatial and temporal resolution is now a great alternative for pre-surgical assessment of CHD including pulmonary venous connections [17]. It gives accurate details of the cardiac anatomy and atypical vessels in 3-dimensional space. The acquired and reformatted images can provide optimum information about vascular course, calibre, defects, and connections and is therefore claimed to be superior to echocardiography. Even with the known radiation risks, computed tomography is among the most efficient methods for imaging that is widely available, can be performed quickly in a single breath hold, and is more economical. We imaged all our cases on a 256-slice, dual-source CT scanner using institutional cardiac CT protocols to keep the radiation risk as low as possible. We used a bolus tracking method for angiograms and used retrospective cardiac gating. Our acquired raw data were processed and reformatted into maximum intensity projection (MIP), multiplanar reformation (MPR), and volume rendered (VR) images for proper evaluation.
MRI is an equally efficient imaging technique for dedicated evaluation of congenital cardiac anomalies and anomalous pulmonary veins [14,18-21]. Many centres with adequate facilities even prefer MRI to MDCTA due to its radiation benefits over MDCTA. It is capable of excellent visualization of cardiovascular anatomy and can play an important role in functional assessment. However, the image acquisition takes longer and requires lengthy anaesthesia; therefore, use of MRI is limited in many centres. It is also costlier and less easily available compared to CT in developing countries.
Partial anomalous venous connections or return (PAPVC/PAPVR)
Partial anomalous venous connections or return (PAPVC/PAPVR) represents an anomaly in which at least one or more PVs, but not all, drain directly into the systemic venous circulation (i.e. right atrium, superior vena cava, or brachiocephalic vein). The prevalence of PAPVC has been reported between 0.4 and 0.7% [22]. The incidence of occurrence is more common on the right side, and it may be detected incidentally on CT or MR imaging [19,23,24]. Partial connections behave like a left to right shunt similar to ASD, VSD, or PDA. Some authors believe that at least 50% of the anomalous pulmonary venous flow in systemic circulation is required to present a clinically significant PAPVC [25].
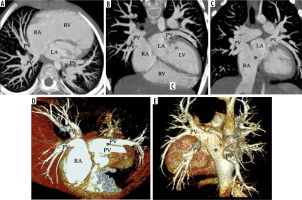
There are different types of anomalous venous connections forming a heterogenous morphological spectrum. These can be described as right sided, left sided, or bilate-ral. Similar to TAPVC, the connections of PAPVC can also be supracardiac, cardiac, infracardiac, or mixed anomalous connections. Of these, the right upper PV draining into the SVC is the commonest type of PAPVC [25,26], which is usually associated with ASD, most commonly sinus venosus type (SV-ASD). The sinus venosus ASD (SV-ASD) results embryologically from a lack of septation between PV and SVC or IVC. In superior SV-ASD, the right upper PV drains into the right SVC or SVC-RA junction, while in inferior SV-ASD the right lower PV drains into the IVC-RA junction when other PVs remain connected to the LA. This conclusive view is supported by Praagh et al. [27]. Other than this, the right-sided PVs may have a supracardiac connection draining into the SVC anywhere between brachiocephalic vein and the SVC, or into azygous, coronary sinus, IVC, or directly into the RA. We are reporting cases of right upper PV with supracardiac connection into the SVC (Figure 1) and superior cavoatrial junction (CAJ) near SV-ASD (Figure 2). Few other PAPVC cases include: right upper and lower PVs with supracardiac connections into the SVC-CAJ (Figure 3) and right upper and lower PVs with cardiac connections into the RA (Figure 4 and 5).
Figure 1
VR images in anterior and posterior view of a patient of right superior pulmonary vein partial anomalous pulmonary venous connection (PAPVC). The images show drainage of right superior pulmonary vein (PV) draining into lower part of superior vena cava (SVC). There is also a single accessary right middle pulmonary vein (*) draining near the cavoatrial confluence. The rest of the pulmonary veins are draining normally into the left atrium
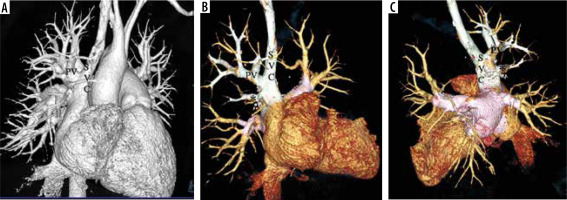
Figure 2
A-B) VR images with clip plane in anterior and posterior view; (C) axial MIP image in a patient of right superior pulmonary vein partial anomalous pulmonary venous connection (PAPVC). The right superior pulmonary vein (PV) drains into superior vena cava (SVC) near the cavoatrial junction. The patient also has a superior sinus venosus atrial septal defect (double headed arrow)
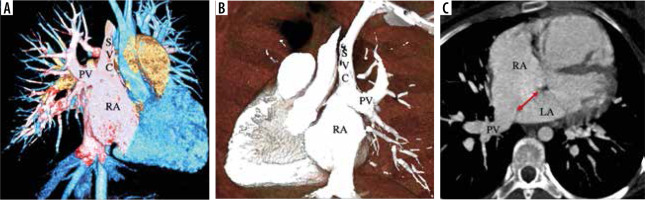
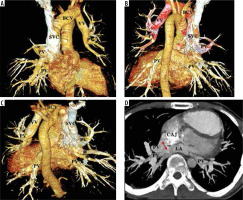
Figure 3
A-C) Thin MIP axial and coronal and (D) VR images from a case of right partial anomalous pulmonary venous connection (PAPVC). The images show a sufficiently sized superior sinus venosus atrial septal defect (ASD). There is right-sided PAPVC with right superior pulmonary vein (PV) draining into the lower part of the superior vena cava (SVC) and inferior pulmonary veins draining at the cavoatrial junction. The left PV is draining normally into the left atrium (LA)
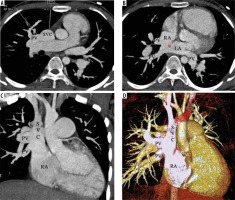
Figure 4
Thin MIP axial and coronal images from a case of right-sided cardiac partial anomalous pulmonary venous connection (PAPVC). The right superior and inferior pulmonary veins (PV) are draining into the right atrium (RA). The left PV have normal connection to the left atrium (LA). An atrial septal defect (*) is also present in superoposterior part with dilated right heart chambers (RA, RV)
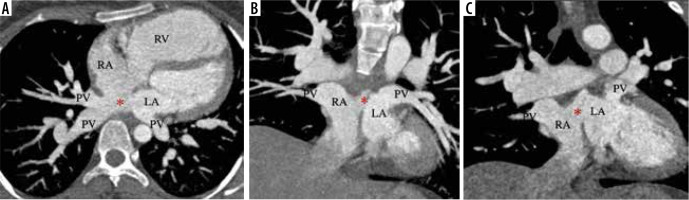
Figure 5
A-C) Thin MIP (axial coronal) and (D, E) images from a case of cardiac partial anomalous pulmonary venous connection (PAPVC). The images show that the right-sided superior and superior pulmonary veins (PV) have a cardiac connection into the right atrium (RA). The left PV are draining normally into the left atrium (LA). A superior sinus venosus atrial septal defect (*) is present with dilated right heart chambers (RA, RV). The VR image compliments the same findings
Amongst the spectrum of PAPVC, left-sided anomalous connections have been reported in nearly 18.2% of cases [16]. The left upper pulmonary vein draining into a vertical vein (VV) and into the brachiocephalic vein is the commonest type [26]. The VV needs to be differentiated from left SVC, which is a common mimic [26]. In normal anatomy, the left upper PV is seen anterior to the left bronchus as a single vein. This vein is absent in left upper PV-PAPVC. However, there are 2 veins when there is a persistent left SVC (SVC and left upper PV). Other sites of left anomalous connections include the coronary sinus and the hemiazygos vein.
Similarly to TAPVC, mixed connections are reported in the PAPVC as well, where at least one PV from each side drains into a different venous compartment. We report a case in which the left upper pulmonary vein drains into the left brachiocephalic vein via a vertical vein, and the right upper pulmonary drains into the CAJ (Figure 6). Anomalous drainage of accessory pulmonary veins are described. In our case, 2 right middle accessory veins drained at the CAJ, at the level of the superior sinus venosus ASD (Figure 6).
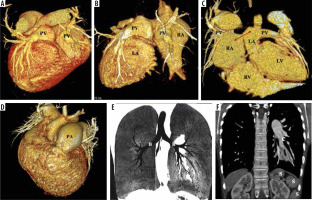
Figure 6
A-C) VR images in different orientation; (D) axial thin MIP image of a patient with mixed type partial anomalous pulmonary venous connection (PAPVC). The right superior pulmonary vein (PV) is draining at the superior cavoatrial junction (CAJ) near the sinus venosus atrial septal defect (ASD) (double headed arrow). The left superior PV is draining into a left brachiocephalic vein (BCV) through an ascending vertical vein (VV). The right and left inferior PV are normally draining into the left atrium. There are also 2 accessary right middle pulmonary veins (*) draining into the LA
PAPVC can be associated with other cardiac congenital diseases. Instead of ASD, other cardiovascular shunts like VSD or PDA can be associated occasionally. Complex cardiac anomalies like heterotaxy syndrome and double outlet right ventricle are often associated with PAPVC. We report a case of right upper PV supracardiac PAPVC with associated PDA (Figure 7) and another case of right-sided PAPVC with cardiac connection as a part of heterotaxy syndrome with left isomerism (Figure 8).
Figure 7
VR images with clip plane in different orientations. The images show partial anomalous pulmonary venous connection (PAPVC) of the right superior pulmonary vein (PV) draining into superior vena cava (SVC). There is a patent ductus arteriosus as shunt (*) [AO – aorta; PA – pulmonary artery]
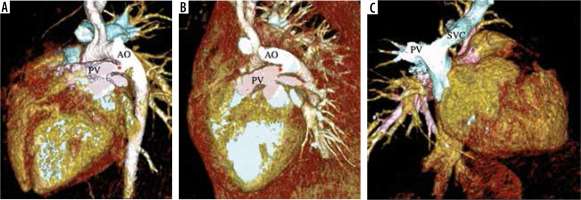
Figure 8
These are images from a case of partial anomalous pulmonary venous connection (PAPVC) associated with heterotaxy syndrome (left isomerism) and pulmonary artery hypertension. A-C) VR images of the heart in posterior orientation and with clip plane showing right-sided PAPVC with a cardiac connection into the right atrium (RA). The left pulmonary veins (PV) are draining normally into the left atrium (LA). A small atrial septal defect is present (double head arrow) with dilated RA and RV. D) VR image showing a dilated pulmonary artery (PA) representing pulmonary artery hypertension. E, F) MnIP coronal and thin MIP coronal images show left bronchial pattern (B) and polysplenia (S)
Pathophysiology and clinical presentation
Irrespective of the type or spectrum, all PAPVCs cause increased volume load in the right heart with increased pulmonary blood flow due functional left to right shunts. This leads to recirculation of the oxygenated blood into the systemic through the pulmonary circulation along with enlargement of right atrium and ventricle. The number of anomalous veins, the size and site of draining ostia, and the presence and size of septal defects are the most important determinants of the haemodynamic load and therefore the clinical presentation.
In the patients with PAPVC, the RA pressure is usually less than the LA, creating a greater compliance for venous drainage. Therefore, until pulmonary venous resistance is equal in both the lungs and there is no pulmonary artery stenosis, venous drainage is always higher on the anomalously draining side or the right side. Single anomalous PV with intact septum drains nearly 20-25% of total pulmonary blood flow while anomalous connection of an entire lung increases this up to approximately 66% of pulmonary blood flow. However, in the presence of a septal defect, there may be a larger left to right shunting as a result of 2 co-existing shunts [28].
Most of the PAPVC cases are detected incidentally because patients are usually asymptomatic or present with mild clinical symptoms. Cyanosis is not a common symptom, when present, and usually occurs in the 3rd-4th decades of life due to increased pulmonary vascular resistance and evident pulmonary hypertension. PAPVC is considered as a treatable cause of pulmonary artery hyper-tension [29].
Scimitar syndrome and scimitar variant
Scimitar syndrome is also termed as hypogenetic lung or pulmonary venolobar syndrome. It is a rare complex anomaly that consists of a conglomeration of findings: right-sided anomalous pulmonary venous drainage, hypo-plasia of the right lung, hypoplasia/aplasia of the right pulmonary artery, and cardiac dextroposition [29]. Systemic arterial collateral supply to the affected lungs from the aorta or other systemic branches are usually present. The anomalous right PV may drain in the supra- or sub-diaphragmatic IVC, the azygous vein, the hepatic or portal venous system, or the RA [19,30], but drainage in the subhepatic IVC is most common [31]. Left-sided cases have also been reported in the literature [32].
The cases presenting as scimitar but without all the typical features of the syndrome are included in scimitar variant. Another mimic where similar findings are associated with an anomalous highly tortuous vein that drains into the left atrium instead of IVC projecting a false positive scimitar sign on chest radiograph, is termed as pseudo-scimitar syndrome. This vein has been described as a pseudo-scimitar vein or a meandering right pulmonary vein [28].
Conclusions
Partial anomalous pulmonary venous connections are uncommon congenital anomalies. Depending on the severity of anomalous drainage, the site of connection, and associated defects, this may be detected incidentally or present with variable symptoms like the other CHDs. MDCTA and MR angiograms are excellent imaging techniques for accurate visualization of the anomalous anatomy in 3-dimensional space and provide optimum description of the disease, which helps the clinical in decision-making and planning of definitive management.


