Introduction
The incidence of renal cell carcinoma (RCC) has been steadily increasing over the past two decades, primarily due to advancements in medical imaging [1,2]. These technological improvements have led to nearly 50% of RCC cases being detected incidentally [3]. As a result, most patients are diagnosed at an early, asymptomatic stage, which significantly enhances their prognosis [4]. Currently, partial nephrectomy is the gold standard for treating small renal masses [5]. However, the rise in incidental findings of small RCCs has introduced various treatment dilemmas, particularly for frail, highly comorbid patients who are often unsuitable for surgery. This scenario also poses challenges for patients with solitary kidneys with filtration function at risk or those who prefer not to remain under active surveillance and seek a minimally invasive curative approach.
Over the past few decades, new nephron-sparing treatment options have been developed, including ablative therapies. Among these, cryoablation has shown the most favourable efficacy and safety profile [6]. Cryoablation offers similar oncological outcomes to partial nephrectomy, with slightly higher recurrence rates but greater safety and the potential for repeated procedures [7-9]. In this article, we share our institutional experience in treating RCCs with percutaneous cryoablation (PCA) gained over one year.
Material and methods
The procedures were conducted at Wroclaw Medical University Hospital, commencing in June 2023, shortly after the allocation of public funding for the PCA procedure. Eighty-one consecutive procedures in 74 patients were performed from the beginning of June 2023 to the end June 2024. Procedures were performed by an interventional radiologist (IR) proficient in computed tomography (CT) interventions (M.G.) and a urologist with vast experience in ultrasound (US)-guided percutaneous renal procedures (percutaneous nephrolithotomies and biopsies) (W.K.).
Patients unsuitable for partial nephrectomy (PN) due to anaesthesiological or surgical reasons with lesions up to 5 cm were enrolled for PCA. Additionally, those who strongly opposed the traditional surgery despite proper counselling were included. Patients with cystic tumours or tumours positioned in a way that prevented safe needle placement (e.g. complete retrorenal colon) were excluded. Other potential exclusion criteria, such as contraindications to CT imaging, local anaesthesia, and sedation were not encountered in this cohort.
Results
Biopsy
Before being deemed eligible for the PCA procedure, the malignant character of the tumour is to be confirmed by the tumour biopsy. It is performed in every primary renal lesion and further management is based on the pathology report. Our protocol allows the biopsy to be omitted in two particular instances: first, when the residual tumour is visualised in the surgical bed following PN; second, when there is evident local recurrence in the retroperitoneal fat or abdominal wall after the surgical approach. Moreover, if the pre-procedural biopsy is not feasible due to logistical or technical reasons, an alternative approach is available. In patients with a strong suspicion of malignancy on imaging, indicated by a rapid growth ratio or a malignant pattern of contrast enhancement, tissue sampling may be performed at the time of PCA, just after placement of cryoprobes.
Preparation for the biopsy is the same as for any other percutaneous renal procedure. It requires a complete blood count (CBC), coagulation assays, an electrolyte panel, renal function assessment, and a urine culture. Because of the high risk of haemorrhage, antiplatelet and anticoagulant drugs are discontinued according to general principles. Nevertheless, no blood products are secured. In the case of a negative culture, one dose of second-generation cephalosporin prophylaxis is given. When asymptomatic bacteriuria is discovered, the patient receives a full course of targeted antimicrobial therapy, starting at least 24 hours before the procedure. Symptomatic urinary tract infection is an absolute contraindication to the procedure.
The biopsy is performed with an 18/20 G coaxial needle, under local anaesthesia, taking 3-8 samples from the different parts of the tumour under hybrid US and CT imaging. When technically feasible, samples are taken from the peripheral part of the tumour, thereby lowering the risk of necrotic tissue sampling. Occasionally, technical difficulties require the use of different sizes of needles or a biopsy gun with varying needle penetration. Additionally, fine needle aspiration material is taken for cytological assessment.
After the procedure, the patient is instructed to maintain a supine position for a few hours. Following this, US and CBC are performed to exclude the formation of clinically significant haematomas. No additional medication is given, except for simple analgesics on demand. If no bleeding is confirmed, the patient is discharged the same day.
PCA procedure
Unless comorbidities indicate otherwise, the patient is admitted to the hospital on the day of the procedure. To ensure safe conduction of the procedure, the following are necessary: urine culture, electrocardiogram, chest radiograph, CBC, creatinine with estimated glomerular filtration rate (eGFR), electrolyte panel and coagulation panel. The antimicrobial prophylaxis does not differ from that applied in a biopsy setting. Also, the antiplatelet and anticoagulation therapy is modified according to the aforementioned principles. However, in this case two units of packed red blood cells (pRBCs) are secured.
Patients are typically sedated using intravenous remifentanil, while local anaesthesia is implemented using lidocaine and bupivacaine according to established dosage regimens. For small, non-complex lesions in cooperative patients, the procedure can be performed solely under local anaesthesia with constant anaesthesiologist supervision. In certain justified cases, general anaesthesia might also be employed. However, considering that the average patient referred for PCA is severely comorbid, there may be instances where even sedation is not feasible. In such cases, if the patient is determined and the procedure offers a potentially curative outcome, it may be possible to proceed under local anaesthesia with ongoing anaesthesiologic supervision. Data from the Surgical Satisfaction Questionnaire 8 (SSQ-8), completed by patients at multiple time points, along with the Numeric Rating Scale (NRS), indicate that the freezing process is generally painless, except during the needle positioning stage. Nevertheless, in some cases, acute pain may occur due to the contact of the iceball with certain nerves. In our experience, iceball growth in the subcostal region and in deep paraspinal muscles might cause significant discomfort. In order to minimise the risk of unfavourable sequelae of haemorrhagic complications, the PCAs are performed in a department with immediate access to endovascular embolisation procedures.
As mentioned before, we perform PCAs under hybrid imaging combining US and CT; the former system is Arietta 60, Fujifilm (once Aloka-Hitachi), Minato City, Tokyo, Japan, and the latter, CT system designed for interventional radiology is Aquilion One, Canon, Tokyo, Japan. The following CT parameters have been used: 0.5 mm collimation, 1 pitch, 0.275 seconds rotation time, 2 mm reconstructed section thickness, 80-120 kV tube voltage, 50-250 mA tube current-time product, tilt 0.0, FOV 50 cm. To limit the radiation dose as much as possible the voltage was often reduced to 80 kV, and the scanning range was restricted to only a narrow region of interest – normally 10-12 cm. Because of the countermeasures mentioned above, the mean and median effective radiation dose were 12.57 mSv and 10.76 mSv, respectively.
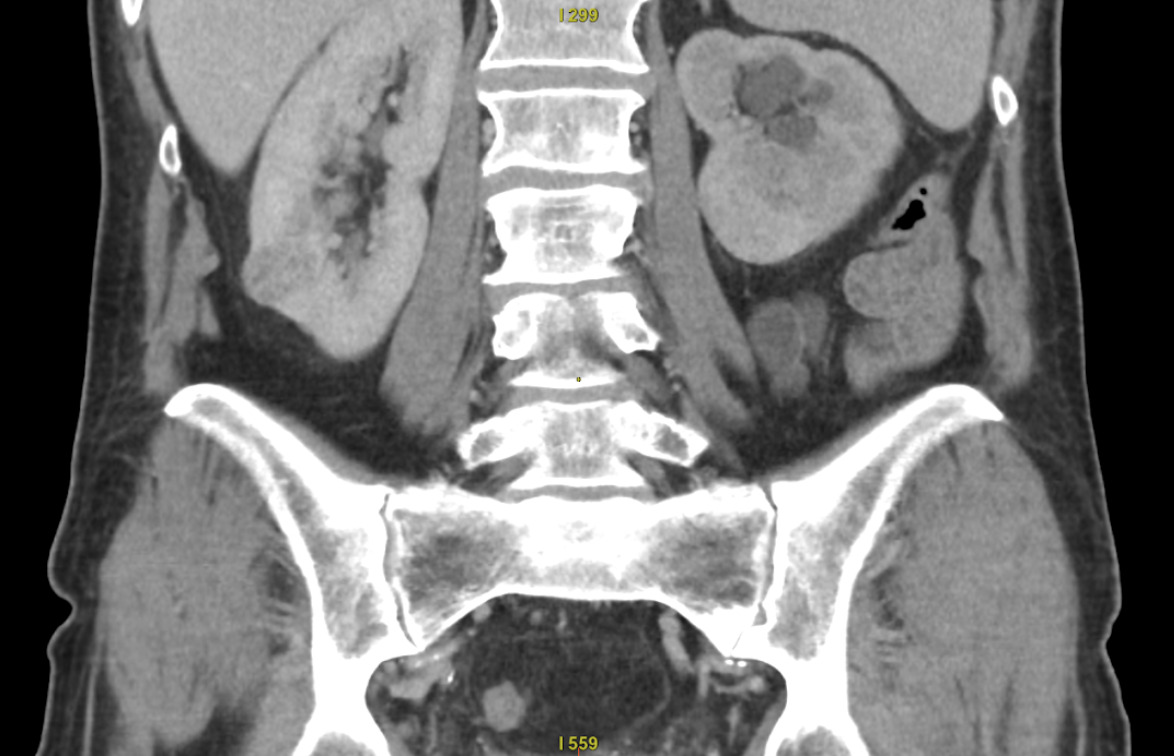
The patients are positioned in the CT scanner to ensure the safest and most direct percutaneous access to the tumour. The majority are operated on in a prone position, as this provides easier CT navigation and facilitates cryoprobe stabilisation. However, sometimes multiple position adjustments using dedicated positioners are needed to achieve a favourable relation between the cryoablated tumour and vital organs. Preliminary US and CT scans are obtained to plan the procedure at various stages of respiration, as the kidney position can significantly change with the respiratory movements (Figure 1). After establishing the anticipated entry site, the area is prepared and draped in a sterile manner. A 2% solution of lignocaine (15-25 ml) is then administered as a local anaesthetic.
First, a “searching” thin wall Chiba 22 G needle is placed under hybrid US and intermittent CT guidance to establish the proper trajectory. Because of this needle’s fineness, clinically significant damage to the tissues is unlikely. It has to be highlighted that the kidney is highly mobile, both due to respiration and passively when in contact with the needles. Therefore, in many cases, the puncture is performed during a specific phase of a respiratory cycle (e.g. on deep inspiration).
The position of the cryoprobes is planned in relation to the searching needle to ensure that the iceball covers the entire lesion with an additional safety margin of at least 5 mm. Tumours located in the proximity of structures that could be associated with the cold-sink effect, such as the renal hilum or liver, require denser cryoprobe coverage and a wider safety margin. When necessary, adjacent structures are relocated by hydrodissection with cold saline solution. It is performed using a 22 G needle and may include contrast medium. The amount of fluid that has to be injected to achieve a safe distance varies and can be as high as 200-300 ml. Dissecting fluid is cooled down before injection, thereby avoiding a cold-sink effect that could reduce the cryoprobes’ freezing efficiency. In addition, we observed that cold fluid causes bowel spasms. If possible, it is recommended to perform hydrodissection after the cryoprobes’ placement, since the presence of abundant perirenal fluid can significantly impair the quality of CT images.
If biopsy was not performed preoperatively, a coaxial biopsy needle is now placed under CT guidance to obtain samples. Also, if the IceCure system is used, biopsy can be performed through the cryoprobe introducer sheath. It is recommended to perform biopsy after cryoprobes’ placement, as the tumour sampling typically causes blood extravasation, significantly impairing CT image quality.
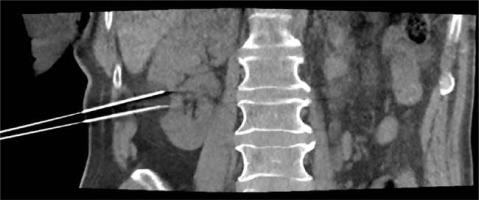
Each PCA is performed with a double freeze-thaw cycle, consisting of at least 8 min of freezing and 8 minutes of thawing. If the clinical circumstance requires it and procedure safety is not compromised, the cycle may be extended up to 15 min. During the freezing cycles, sequential CT scans are conducted every 5 min in order to monitor the growth of the iceball (Figure 2). The main aim of the procedure is to cover the whole lesion with the iceball with an additional margin [10-12]. However, due to the variable properties of different tissues, predicting the iceball’s growth rate and final shape is challenging. When the distance to vital organs is minimal, more frequent CT monitoring is necessary. To avoid catastrophic consequences, the threshold for halting further freezing should be low, especially when the bowel is near the iceball margin. In our protocol, the safety of the bowel, ureter and pleura is prioritised over oncologic radicalness.
When the lesion is not covered after the planned freezing time, additional freezing time might result in iceball growth in some cases. If not, more cryoprobes ought to be positioned [13]. Needles are removed as soon as they thaw, and forceless extraction is possible. It is of utmost importance to properly stabilise the cryoprobes during the procedure and not to force cryoprobe extraction, as it might cause kidney injury on the ice border and lead to significant bleeding. The patients must be instructed not to move extensively. If not possible, deeper sedation is required. The final CT scan is performed a few minutes after removal of cryoprobes. When abundant blood extravasation is visualised, further CT scans are performed to assess the final form and volume of the hematoma. If significant bleeding is suspected, angiography with or without embolization might be indicated. Finally, the pressure dressing is applied, and the procedure is finished.
Similarly to the tumour biopsy, after the procedure, patients are instructed to remain supine for a few hours. Subsequently, a US scan and CBC are performed to exclude formation of clinically significant hematomas. No additional medication is given, except for simple analgesics on demand. If no bleeding is confirmed, the patient is discharged on the same day.
Discussion
Starting a cryoablation programme is a considerable organisational challenge. In our centre, it became possible after receiving public funding for the procedure. In our opinion, especially in hospitals that do not allocate dedicated beds for interventional radiology, close cooperation between IRs and urologists is a vital factor for PCA programme success. Departments responsible for coordinating PCA programmes must allocate beds for postprocedural overnight stays and managing any potential complications. In our setting, when such a necessity arose, individuals were admitted to the urology department
As PCA is often performed as an imperative procedure in severely comorbid patients, it is a difficult procedure with a flat learning curve. The responsibilities of a urologist include proper patient selection for the procedure, preoperative preparation, and postoperative care, including treatment of most complications. On the other hand, the radiologist must organise the CT operating room (CT-OR). This includes ensuring that the operating physicians and the anaesthesia team are involved, along with at least one surgical nurse, and a team of technicians. Due to the nature of the procedure and the frequent re-entry to the operating room after CT scanning, each team member must have clearly defined responsibilities. Only good teamwork will allow for the efficient execution of the procedure in optimal time without radiation exposure. In our setting, specialists have gained their experience concurrently over years of practice. The urologist has developed expertise in renal punctures through the performance of numerous percutaneous nephrolithotomies, while the interventional radiologist has gained skills through renal biopsies and other interventional procedures. Also, in order to facilitate efficient cooperation, the operators (M.G., W.K.) completed two training sessions at reference centres before starting the PCA programme.
In our opinion, anaesthesiological supervision of the procedure is vital, as these patients are often frail and severely comorbid. Additionally, direct access to angiographic procedures is compulsory, because of the risk of significant peri- and postoperative bleeding. Before starting the programme, it is also necessary to prepare appropriate CT software protocols that will allow for optimal imaging in a small field with numerous artefacts resulting from the presence of metal needles.