Introduction
The breast glands are the largest secretory structure in the human body. They are built of glandular, fat, and connective tissue. The glandular tissue is organised in 15-25 lobules, arranged radially around the mamilla and separated from each other by connective tissue. Every lobule is an individual alveolar gland with its own lobar lactiferous excretory duct. Before the entrance to the mamilla, the ducts expand and create a lactiferous sinus, which during lactation acts as a reservoir of food, after that period, they narrow again so that every duct has its orifice in the nipple [1].
Due to the presence of oestrogen and progesterone receptors, the glandular tissue is sensitive to these hormones throughout the menstrual cycle. The number of oestrogen receptors is lower during the luteal phase, whereas the number of progesterone receptors remains fairly constant. Oestrogens affect the proliferation of the epithelial cells and bind to the nuclear receptors ERα and ERβ. The most intense mitotic activity of cells in the entire breast gland was found during the luteal phase when the concentration of progesterone was the highest. The largest dimensions of the mammary gland are achieved at the end of the luteal phase [2].
The aim of the study is to present the influence of hormone levels in a woman’s life (depending on menstrual cycle, pregnancy, and menopause) on ultrasound images of the breast gland.
The development of the mammary gland
The development of the mammary gland begins intrauterine with the appearance of small glandular tissue clusters that later develop to form mammary glands made of around 15-24 lobules. The following stage occurs during puberty, which leads to the final maturity of the gland over the reproductive period and is affected by changes in hormone levels throughout the menstrual cycle until menopause. Moreover, from around the age of 35 a steady involution of the lactiferous ducts occurs [3]. The terminal extralobular duct together with the intralobular duct and the secretory vesicles flowing into it constitute the basic functional unit of the gland, called the terminal duct lobular unit (TDLU) (Figure 1).
During the intrauterine period, the development of mammary glands is independent of hormones. It begins with a thickening on the thoracic wall and ends after birth with the formation of 2 nipples and the emergence of a lactiferous duct system.
Hormone receptors on the glandular cells grow in numbers directly before puberty, which leads to the assumption that the role of hormones in the development of the mammary gland is most crucial from the start of puberty. The process starts with the lengthening of the lactiferous ducts and the formation of new side branches during the upcoming menstrual cycles. Each subsequent pregnancy increases the development of side branches and induces lactation [4].
The primary role of oestrogens and progesterone is to stimulate the mammary glands during the menstrual cycle. On the other hand, during pregnancy and lactation the glands mainly respond to the change in levels of prolactin (PRL) and growth hormone (GH). The adrenocortical glands also participate in this process, but their role is only supplemental and limited to producing hydrocortisone and adrenal androgens [5].
In puberty, ovaries begin oestrogen secretion, causing the accumulation of fat cells in the connective tissue of a lactiferous line. Due to this the mammary glands grow and a system of lactiferous ductulus and ducts develops; this process usually happens at the same time as the appearance of pubic hair. Next, the start of menstruation and ovulation promotes the formation of secretory glands at the end of lactiferous ducts, the lactiferous ductulus and ducts grow further, and the development of glandular tissue and lobules proceeds [6] (Table 1).
Table 1
Stages of mammary gland development during puberty
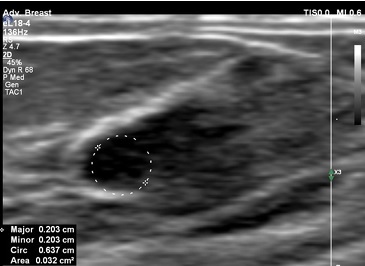
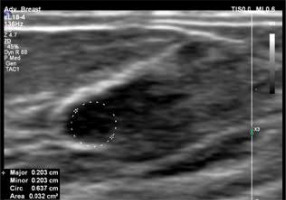
In the menstrual cycle, oestrogens are produced mainly through the ovaries during the first phase of the cycle and stimulate the growth of lactiferous ductulus in mammary glands. After ovulation, during the second phase of the cycle, progesterone plays a dominant role in stimulating the lactiferous gland formation. Many women can observe rapid changes in the structure of their breasts such as oedema, pain, and asymmetry. During this cycle, the mammary glands prepare themselves for possible pregnancy and will return to their standard size if fertilisation does not occur [7] (Figure 2).
Figure 2
US of the mammary gland during the luteal phase of the cycle with a visible growth of the lactiferous ductulus
During menopause, there is an imbalance between the levels of oestrogen and progesterone. The oestrogen level drops greatly, dehydrating the mammary glands connective tissue, leading to the loss of flexibility and form. The result of these changes is so-called “‘saggy breasts” [8].
Physiological changes in the ultrasound image of the breasts during menopause most often result from modifications in the shape and arrangement of the mammary ducts. This is an expression of the aging of the tissues inside the breast and may cause symptoms such as a lump behind the nipple or discharge from the nipple. Another quite common change during menopause is the formation of cysts. They may cause swelling and local pressure pain, but these changes are rarely life-threatening. They usually go away on their own, and sometimes modifying hormonal treatment is beneficial if a woman is undergoing menopausal hormone therapy [9,10].
Periodic, transient breast pain during menopause is usually related to the aging of the mammary gland, clearly visible in the ultrasound image as atrophy of glandular tissue. This type of pain co-occuring in both breasts usually excludes the presence of cancer [11,12].
Hormones work by a feedback mechanism; they regulate each other’s synthesis and secretion. Oestrogens actively affect the hypothalamus, which in effect stimulates the pituitary gland to increase the production and secretion of prolactin. Prolactin controls the activity of the corpus luteum in the ovary, which stimulates the production of progesterone, inducing a transcription of oestrogen receptors in many tissues. Consequently, it is not possible to evaluate clearly, in physiological conditions, whether the changes in mammary glands are caused by changes in hormone levels or are a result of a secondary influence by the stimulation of other organs and structures [13].
Oestrogens stimulate ERα receptors, expressed in the cells of mammary epithelial tissue and stroma. The epithelial tissue containing these receptors, under the influence of oestrogens, grows, filling the fat tissue in the mammary gland with side branches. The substantial proliferation of cells is observed mainly at the end of lactiferous ducts, which grow and form into the shape of a spoon, known as ending buds [14].
The progesterone receptor is expressed not only by the cells of epithelial tissue but also by the mammary stroma. It is important to remember that the role of progesterone in the development of mammary glands is not as crucial as that of oestrogens. Therefore, even in the absence of progesterone, the development of the mammary gland remains undisturbed. Nevertheless, we can observe some adverse effects of this hormonal imbalance, such as a lack of side branches of the lactiferous ducts or lactiferous follicles [15].
Prolactin plays no relevant role in the process of forming the mammary glands nor in their rebuilding during the menstrual cycle. Its primary role occurs during pregnancy when the oestrogen and progesterone growth of the lactiferous ducts and side branches stimulate its release. Prolactin stimulation is required for the correct formation of the lactiferous follicles and differentiation of cells; here we can observe typical morphological features such as the lactiferous cells’ fat drops and “grainy” cytoplasm [16].
The formation of mammary glands is strictly controlled by oestrogens, progesterone, and prolactin, sequentially impacting epithelial tissue in synergy with corticosteroids and GH, which probably works on epithelial tissue and mammary stroma by a GH receptor (GHR) [17].
During puberty, the level of oestrogen rises, preparing epithelial cells for the influence of progesterone by inducing the expression of progesterone receptors. The cyclical secretion of progesterone in the menstrual cycle takes place after puberty simultaneously as the lactiferous ducts reach the edge of the fat tissue of a mammary gland with the side branches [18].
The sequential effect of each hormone allows an orderly course of the mammary gland’s formation. Essentially, all the ducts are arranged in front of the lactiferous follicle’s buds and have adequate space for effective drainage. This sequential occurrence of each stage of development of the mammary gland, which manifests by the expression of various hormone receptors, is highly correlated with the presence of those hormones at a given time. The main complication is that each hormone is found both in blood and glandular tissue in various concentrations, they impact each other on many different levels, and are strictly controlled by the hypothalamus–pituitary axis [19].
Physiological changes in mammary glands during pregnancy and lactation
Changes in oestrogen, progesterone, and prolactin serum concentration induce physiological changes in the breast’s architecture, which are also visible during a histologic examination and lead to the increase of breast density, especially in young women [20].
In the first trimester, the proliferation and growth of lactiferous ducts and to a lesser extent the growth of follicles and lobules begin under the influence of oestrogens. The hyperplasia of glandular tissue leads to a gradual invasion of fat tissue, along with the increase of vascularisation and blood flow [21].
In the second and third trimesters, progesterone induces the hyperplasia of lobules and an involution of fibrous and fat stroma. Usually, the largest growth of the breast gland occurs until the 22nd week of pregnancy (Figures 3 and 4).
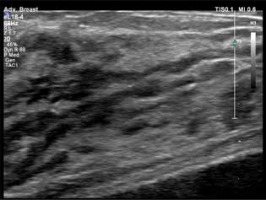
Figure 3
US of the mammary gland during the first trimester of pregnancy – the gland’s parenchyma is mostly hypoechoic and the widening of the lactiferous ducts is visible
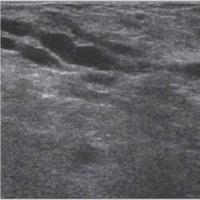
Figure 4
US during the second trimester – the mammary gland’s parenchyma shows higher echogenicity and the widening of lactiferous ducts is clearer than in the first trimester
At the end of pregnancy, the high levels of oestrogens and progesterone counteract prolactin, inhibiting the production of milk; the production of colostrum, however, remains undisturbed. The reduction of oestrogen and progesterone levels in the postnatal period leads to the release of prolactin, triggered by the hypothalamus. In this period, lactation is also supported by the physical stimulation of nipple by the neonate, which activates the secretion of oxytocin by the anterior lobe of the pituitary gland as a way to maintain lactation [17].
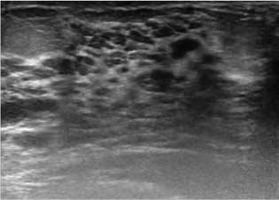
After labour, lactogenesis occurs; it is the transformation of the glandular tissue from the state of proliferation to secretion (Figure 5). As a result of these changes, the typical image in ultrasonography is hypoechogenic due to the hyperplasia of glandular tissue, but it changes readily into a hyperechogenic image during the lactation period due to increased vascularity and bulging of the lactiferous ducts [22].
Figure 5
US during the lactation period, diffused hyperechogenic parenchyma with the widened lactiferous ducts as an effect of milk accumulation
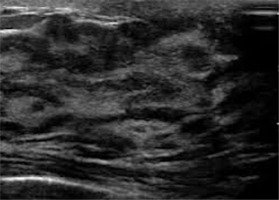
Clinical and physiological changes in the mammary glands manifest themselves in mammary glands progressing growth, firmness, and protuberance, making a clinical exam difficult. In a population of breast-feeding women, it was found that the location of fat and glandular tissue is different between women but not between both mammary glands of each female.
The average number of the main lactiferous ducts is 9.6 ± 2.9 for the left mammary gland and 9.2 ± 2.9 for the right. The average diameter of the main ducts, located at the base of the mamilla, is 1.9 ± 0.6 mm for the left breast and 2.1 ± 0.7 mm for the right breast. The percentage of glandular and fat tissue in the entire mammary glands structure in the ultrasonographic image is 63 ± 9% and 37 ± 9% for the left mammary gland and 65 ± 11% and 35 ± 12% for the right. Milk production does not correlate with the quantity of glandular tissue, the number of lactiferous ducts, the average diameter of main ducts, or the storage capacity of mammary glands [23].
The blood flow in the mammary glands measured by pulsed-wave Doppler is increased mainly by the branches of the internal and lateral thoracic arteries, which are responsible for, respectively, 60-70% and 30% of the blood flow. The increased blood flow persists during the lactation period and does not return to its previous levels until 2 weeks after the lactation [24].
The colour Doppler function to evaluate women during lactation showed that the blood flow varies significantly between females. However, it remains comparable between the 2 mammary glands of each patient [20].
Although there is no evidence of a connection between the blood flow in the mammary glands and milk production, the decrease in blood flow in women with low milk production during lactation suggests the existence of a blood flow threshold, under which milk production is impaired [20].
Conclusions
The image in breast ultrasound examination depends on the phase of the woman’s cycle and individual hormone levels. During hormonal disorders, such as irregular menstrual cycle, secondary amenorrhoea, or menopause, the described hormonal changes should always be considered when assessing the breast ultrasound image.