Introduction
Renal cell carcinoma (RCC) ranks 6th in men and 10th in women as the most common newly detected malignancies [1]. One-third of patients with RCC will develop bone meta-stases, mostly spinal (15%), which may be symptomatic even before the site of neoplastic origin is diagnosed [2]. Skeletal metastases worsen the prognosis – more so for the axial bones than for extremities – with an average life expectancy of 12-24 months [3]. Symptoms of spinal meta-stases include intractable pain and neurological deficits due to direct compression, pathological fractures, or instability [4]. Promising results of implementation of immunotherapy agents such as tyrosine kinase inhibitors led to a paradigm shift in the therapy of metastatic RCC in which systemic therapy has been the primary strategy for the past several decades [5]. However, due to notorious resistance of metastatic RCC, surgical decompression of neural structures and instrumental stabilisation of vertebral column are often necessary [6]. Laminectomy alone may induce spinal instability and does not eliminate pain related to axial loading of the tumour-infiltrated spine [7]. Thus, the optimal surgical treatment options are: vertebrectomy if feasible, posterior decompression with fixation, and cement augmentation of pathologic fracture [8].
As for many other tumour entities, arterial embolisation is a common preoperative adjunct for removal of these highly-vascularised lesions [9]. Although the first description of the procedure was published back in 1975, its risks and benefits remain controversial [10]. While some studies report a decrease in intraoperative blood loss, the opposite effect has also been observed [11-13]. That is why, to date, there is no consensus regarding the procedure; although some centres implement it routinely, others do not refer patients for preoperative embolisation.
The objective of the present study was to analyse the usefulness of arterial embolisation, its complications, and its impact on intraoperative blood loss and neurological outcomes.
Material and methods
Study population
We retrospectively identified all patients who underwent embolisation followed by surgical decompression and instrumental fusion for spinal metastatic RCC in our institution between 2016 and 2023. The inclusion criteria were defined as follows: 1) histopathological evidence of metastatic RCC, 2) (potential) instability of the spine or spinal cord compression, and 3) surgery following the endovascular treatment within 24-48 hours. Patients with tumour entities other than RCC were excluded from the study. The study, carried out in a hospital serving a population of 2.1 million people, was approved by the local Ethics Committee and was conducted according to the Declaration of Helsinki. All patients (or their legal relatives) signed an informed consent form.
Clinical data collection
The following data were collected: sex, age, level of embolised spinal metastasis, embolising agent, extent of obliteration, complications, and intraoperative blood loss. The level of the target lesion was determined based on imaging examinations (computed tomography and/or magnetic resonance imaging).
Endovascular procedures
All interventions were performed by interventional ra-diologists with more than 5 years’ experience in endovas-cular embolisation. Procedures were conducted in an in-hospital regimen under local anaesthesia or analgosedation depending on the patient’s condition. In Angiosuite, via femoral access, first a panoramic aortic DSA above the targeted level was acquired to visualise arterial feeders. Afterwards, selective injections were carried out depending on the feeders’ origin; the endovascular strategy was customised case by case based on the angioarchitecture. Superselective embolisations were achieved; the type of microcatheters and embolics depended on local availability and operator prefe-rences. Technical success was defined as complete exclusion of the vascular supply or occlusion of > 75% of the lesion at the last DSA run, with noticeably slower blood inflow.
Procedural complications were evaluated according to CIRSE classification system [14]. A mechanical closure device or manual compression of the site of puncture were applied based on the centre’s experience and the patient’s anatomy.
Surgical procedures
All patients were operated by experienced orthopaedic surgeons using standardised techniques within the first 24-48 hours after embolisation (on the day following the endovascular treatment). In urgent cases, surgical procedures were performed on the same day. The surgical approach was dependent on the anatomical conditions (lesion level, extent of site osseous destruction and soft tissue tumour) and included anterior decompression followed by stabilisation and laminectomy with posterolateral decompression. During the procedures, intraoperative blood loss (IBL) was estimated.
Results
In total, 59 patients with a mean age of 63 years (range from 32 to 81 years) were included. The majority of patients (78%) were male. All the patients were in renal cancer stage 4 according to the American Cancer Society (ACS), with at least one bone metastasis. Lesions were located in cervical (one patient, 2%), thoracic (25 patients, 42%), and lumbar spine (30 patients, 51%). In 3 cases (5%) lesions were located in both thoracic and lumbar levels.
Complete embolisation, defined as complete exclusion of the vascular supply or occlusion of > 75% of the lesion, was achieved in 76% (45/59) and partial embolisation in 15% (9/59) of cases. In 5 cases (8%) safe occlusion was not possible due to the radiculomedullary artery originating from the same pedicle as the tumour (Figure 1). The me-dian number of embolized arteries was 2 (range from 1 to 6).
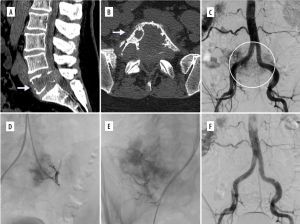
Figure 1
Two cases in which radiculomedullary artery (white arrows) originated from the same pedicle as the tumour. In both patients, embolisation was not performed
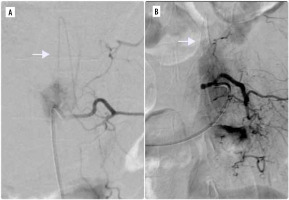
In terms of embolic materials, microspheres with size ranging from 200 μm to 900 μm were the most commonly used embolic material. In 10 cases (17%), microcoils were used in addition to the microspehres (Figure 2). In one patient, Glubran (tissue glue) was used.
Minor complications (vomiting, increased pain) occurred in 8 patients. All patients received additional medica-tion and were further monitored for 24 hours. As far as serious complications were concerned, paraplegia (one transient and one permanent) was noted in 2 cases.
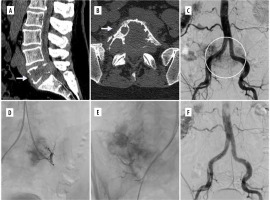
Figure 2
Example of a renal cell carcinoma metastatic lesion causing destruction of L5 vertebra. A, B) Preoperative CT images in sagittal and axial views. C) Initial DSA image showing aortography with large vascular blush in the region of clinically known lesion. D, E) Selective catheterisation and embolization with coils and microspheres was performed. F) Control DSA disclosing complete occlusion of the feeding vessels
Overall, estimated IBL was 830 ± 410 ml (range from 150 ml to 4700 ml). More than 1000 ml IBL was noted in 16 patients (27%), and less than 1000 ml intraoperative blood loss was detected in 43 patients (73%). Nineteen patients (32%) required intraoperative blood unit administration. The patients were divided into 2 groups: complete embolisation (CE) and incomplete embolisation (IE). As mentioned previously, the majority of patients (73%) were included into CE. In this group, the IBL was lower than in the IE group – 630 ± 380 ml vs. 710 ± 290 ml. Apart from this, IBL in the CE group was lower compared with patients in which embolisation was not performed due to safety reasons (5 patients, 930 ± 180 ml IBL). However, the statistical analysis was not performed due to the relatively small sizes of the incomplete embolisation (9 cases) and no embolisation (5 cases) groups.
Data for clinical details, technique, and completeness of embolisation complication rate as well as IBL in all groups are provided in Table 1.
Table 1
Demographics, and clinical and procedural details of the patients
Discussion
Although intense intraoperative bleeding poses a major risk during the surgical removal of spinal metastatic tumours, high-level evidence on the effect of preoperative embolisation remains a matter of debate. Whereas some authors observe significant reduction of intraoperative blood loss, others report no difference when compared with patients without endovascular treatment [13,15-19]. Some authors underline the role of complete embolisation, which is associated with significantly lower blood loss compared with partial or incomplete exclusion of blood supply to the lesion [16,20]. Despite the lack of the consensus, preoperative embolisation of renal cell carcinoma metastases of the spine was introduced in our centre, and in this study we evaluated the outcome of endovascular procedures and their impact on surgical treatment.
The first key objective of this study was an answer to the question of whether metastatic RCC patients who undergo a preoperative embolisation suffered less blood loss during surgical treatment. We observed lower IBL in cases in which complete embolisation was achieved (630 ± 380 ml vs. 710 ± 290 ml in incomplete embolisation and vs. 930 ± 180 ml with no embolisation). This stays in line with findings made by other authors [16,21,22]. Interestingly, the only prospective, single-blind, and randomised study on preoperative embolisation in surgical treatment of spinal metastases failed to show the blood reduction in embolised patients [18]. However, in their recent paper, Koob et al. hypothesised that the information on completeness of embolisation might be an encouraging factor for a surgeon, which might result in more radical and invasive resections in these patients and relatively higher risk of blood loss [13]. In our experience with pre-operative embolisation of hypervascular lesions in general, we agree that this might be a possible explanation.
Another important factor that might influence the blood loss is the timing of the surgical procedure. Although most authors recommend that the surgery should be performed within the first 24 hours following the embolotherapy, these recommendations are based rather on their experience than scientific reports [15,17,23]. Tang et al. published a comparative study aiming to determine better timing (the same day or the next day) between embolisation and surgery by assessing the safety and efficacy of embolisation [24]. The authors not only failed to show the superiority of the immediate surgical treatment but also suggested to schedule the procedure for the next day in order to allow endovascular complications to be detected and treated so that they would not be misdiagnosed as surgical complications. Based on these findings, in our centre all procedures that could be scheduled for the following day were performed accordingly.
The secondary aim of our paper was to evaluate the feasibility and safety of endovascular embolisation as well as technical and procedural details. In our experience with 59 patients, complete occlusion of the blood supply was achieved in over 75% of cases (45/59). This rate of complete embolisation was comparable with other studies [13,18,21-23,25]. Similarly to other authors, microspheres, coils, and tissue glue were the most common embolics used during the procedure. Partial embolisation, noted in 9 cases (15%), was caused mainly by the small calibre of the feeding vessels or technical limitations precluding safe and distal access. In 5 patients (8%) embolisation was not performed due to the visualisation of the radiculomedullary artery originating from the same pedicle as the tumour and potential risk of the vessel occlusion (see Figure 1).
In terms of major complications, despite thorough evaluation of pre-procedural CT and digital subtraction angiography (DSA) for the detection of possible blood supply to the spinal cord, 2 cases of paraplegia (one transient and one permanent) were observed. Chatani et al. underlined the role of selective CT angiography in the detection of radiculomedullary arteries that were not visible in DSA due to large metastatic mass with destruction and tumour blush of the vertebral body [26]. As far as transient neurological deficits are concerned, some authors attribute them to tumour swelling resulting in spinal cord compression [27]. This is believed to be relevant especially in case of highly vascular tumours with multiple and complex feeding vessel architecture that require long and extensive endovascular treatment. According to some surgeons, immediate decompression might be an efficacious strategy [28,29]. In light of these findings, our patient was administered corticosteroids (dexamethasone 16 mg) and was referred for urgent decompression. Fortunately, the deficit disappeared, and the patient was discharged in good clinical condition.
Our study has certain limitations, which have also been repeatedly emphasised in previous similar studies. Firstly, the relatively small sample group and retrospective character of the study limits the validity of the data. Secondly, the type, extent, and aggressiveness of surgery as well as size of the tumour (rarely indicated in the studies) play a crucial role in IBL. Finally, we did not include a control arm including patients operated without embolotherapy. Nonetheless, as mentioned previously, endovascular embolisation became a standard of care in our centre nearly a decade ago, which is why the data of patients treated differently are lacking.
Conclusions
Although the impact of the endovascular embolisation of renal cell carcinoma metastases to the vertebral column on intraoperative blood loss remains a matter of debate, minimally-invasive embolisation is becoming a standard procedure that is feasible and safe with acceptable rate of complications. Further prospective studies are required to determine the optimal timing of the surgical procedure.