Introduction
Radiological examinations are critical in the evaluation of patients for both diagnosis and treatment. In recent years, technological advancements have resulted in progress in patient diagnosis and treatment. While the number of radiological examination options has grown, new devices and protocols with lower radiation exposure risks for patients have been developed.
Haematological malignancies can easily and quickly destabilize the patient. Clinical and physical examination findings in haematology patients may be hidden by neutropaenia, which may alert the clinician before the patient’s overall state deteriorates. As a result, radiological examinations are frequently used in chemotherapy and/or radiotherapy patients for complications such as neutropaenic fever.
Radiologic examinations such as high-resolution computed tomography (HRCT) are frequently used together with clinical and laboratory tests to show the fever focus. Radiologic examinations are also used to stage the disease and assess the response to treatment in such lymphoma patients.
There are articles in the literature about the effects of radiation exposure from radiological examinations used for diagnostic purposes, particularly in children, on the rest of the patient’s life [1]. Because of the high mortality and short survival rates associated with haematological malignancies in adults, this issue appears to have been overlooked in the literature. Some studies have found that diagnostic radiation exposure does not significantly increase the risk of cancer [2-5], and some findings indicate that the risk may rise, albeit only slightly [6]. There are few studies on diagnostic radiation exposure in patients with haematological malignancies in the literature [7].
According to the NRCP (National Council on Radiation Protection and Measurements), the annual occupational radiation exposure in the United States is 20 mSv/year (on an average of 5 years); no more than 50 mSv in any year and no more than 100 mSv in 5 years [8]. However, sufficient data on the reference value of the radiation dose to which patients with haematological malignancies are exposed could not be found in the literature. Keeping this in mind, it is critical in diagnostic radiology to establish reference values. In the future, these reference values will be useful for comparing data from various haematology clinics to these, as well as for these clinics to raise awareness and self-control so that patients are exposed to less radiation.
The aim of the study was to contribute to the literature by determining the radiation exposure of 3 different types of haematological malignancies (diffuse large B-cell lymphoma [DLBCL], acute myeloid leukaemia [AML], and multiple myeloma [MM]) in the first year following diagnosis. This is the first report to go into detail about the subject of concern. There were no similar studies found after a thorough search.
Material and methods
The number of patients diagnosed pathologically with DLBCL, AML, and MM between 2016 and 2019 was calculated retrospectively. Before the study, approval was obtained from the local Ethics Committee (30.11.2021/number 21).
With the help of the biostatistics department, power analysis was carried out. The study included 43 patients randomly selected from DLBCL patients, 46 patients randomly selected from AML patients, and 55 patients randomly selected from MM patients. Informed consent was obtained from patients who participated in this study. It documented the descriptive characteristics of these patients at the time of diagnosis, such as their age, gender, and stage.
Radiological examinations (such as X-rays, computed tomography [CT], positron emission tomography–computed tomography [PET/CT], scintigraphy, interventional radiology, and radiotherapy) performed on these patients within the first year after pathological diagnosis were reviewed retrospectively. Each patient’s radiation dose received within the first year of their diagnosis was calculated. The total and median CED values (cumulative effective radiation dose in millisieverts [mSv]) of each patient were used for this purpose. Furthermore, the data from these 3 different disease groups were to be compared.
Each patient’s total and median estimated CED value (cumulative effective radiation dose) was calculated and recorded in millisieverts (mSv) using a web-based calculator (www.XRayRisk.com). This calculator estimates doses without taking into account weight or age and is based on all imaging modalities and interventional radiology procedures. The doses obtained from each modality (X-ray, CT, nuclear medicine examinations, interventional radiological procedures) and the data from these 3 disease groups were compared (DLBCL, AML, and MM).
Statistical analysis
All statistical analyses were performed using SPSS 25.0 (IBM SPSS Statistics 25 software; IBM Corp., Armonk, NY). Continuous variables were defined by the mean ± standard deviation, and categorical variables were defined by number and percentage. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used for the determination of normal distribution. For independent group comparisons, the Mann-Whitney U test and Kruskal-Wallis variance analysis (post hoc: Mann-Whitney U test with Bonferroni Correction) was used when parametric test assumptions were not provided. The dif-ference between categorical variables was analysed with c2 analysis. Spearman correlation analysis was performed to investigate the relationships between continuous variables. Statistical significance was determined as p < 0.05.
Results
A total of 43 randomly selected DLBCL patients, 46 randomly selected AML patients, and 55 randomly selected MM patients were included in the study.
There was no significant difference between the groups in terms of gender (p = 0.52). The male/female ratio was 17/26 in the AML group, 22/24 in the DLBCL group, and 28/27 in MM group.
The mean age of the patients at the time of diagnosis included in the study was 57.7 ± 17.28 in the AML group, 62.7 ± 14.79 in the DLBCL group, and 64.95 ± 10.89 in the MM group.
When patients with DLBCL were examined at the time of diagnosis, 3 patients (6.5%) were classified as stage 1, 5 patients (32.6%) were classified as stage 2, 17 patients (37%) were classified as stage 3, and 11 patients (23.9%) were classified as stage 4.
When patients with MM were examined, 12 patients (21.8%) were evaluated as stage 1, 13 patients (23.6%) were evaluated as stage 2, and 30 patients (54.5%) were evaluated as stage 3 at the time of diagnosis.
Table 1 shows that patients with DLBCL had higher PET/CT measurements and those with AML had higher CT counts. AML patients had 12.14 ± 10.69 X-rays, 8.08 ± 5.78 CT scans, 1.60 ± 0.55 scintigraphy scans, and 1.22 ± 0.44 interventional radiology. Patients with DLBCL had 9.94 ± 8.41 X-rays, 5.71 ± 4.30 CT scans, 2.78 ± 1.14 PET/CT scans, and 3.25 ± 0.96 scintigraphy scans. MM patients had a total of 7.75 ± 5.21 X-rays, 4.07 ± 2.79 CT scans, 1.24 ± 0.49 PET/CT scans, 1.20 ± 0.42 scintigraphy scans, and 2.00 ± 1.69 interventional radiology scans (Table 1).
Table 1
Counts of examinations involving radiation in one year after diagnosis
According to Table 2, patients with DLBCL received most of their radiation exposure through PET/CT measurements, whereas patients with AML received the majority of their radiation exposure via CT counts. AML patients received radiation doses of 1.47 ± 1.28 for X-rays, 43.49 ± 36.15 for CT scans, 1.35 ± 3.95 for nuclear medicine, and 0.26 ± 0.54 for interventional radiology. Radiation doses for X-rays, CT scans, nuclear medicine, and interventional radiology were given to patients with DLBCL at 1.39 ± 1.79, 27.29 ± 27.65, 34.20 ± 20.31, and 0.13 ± 0.34, respectively. The X-ray dose for MM patients was 1.10 ± 1.28, the CT dose was 13.48 ± 18.06, the nuclear medicine dose was 13.14 ± 10.35, and the interventional radiology dose was 0.27 ± 0.93 (Table 2).
Table 2
Doses of examinations involving radiation in one year after diagnosis
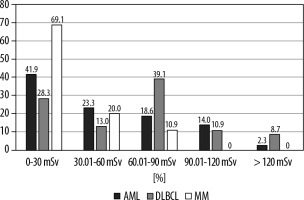
In all 3 haematological malignancies, notably in the DBLCL group, radiation exposure was considerable during the first year of diagnosis. The total radiation dose in one year after diagnosis was 46.54 ± 37.12 (median dose: 36.2) in the AML group, 63.00 ± 42.05 (median dose: 66.4) in the DLBCL group, and 28.04 ± 19.81 (median dose: 26.0) in the MM group (p = 0.0001). There was a significant difference between DLBCL and MM groups. The distribution of AML, DLBCL, and MM patients by dose range of radiation exposure is shown in Figure 1.
Figure 1
Distribution of acute myeloid leukaemia (AML), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma (MM) patients by dose range of radiation exposure
Although there is a negative correlation between the age at diagnosis and the one-year total radiation dose in DLBCL, it is not statistically significant. A negative correlation was observed between the age at diagnosis and the one-year total radiation dose in MM, which is statistically significant (r = –0.298, p = 0.027). The negative correlation between the stage and the one-year total radiation dose in DLBCL is also statistically significant (r = –0.306, p = 0.038).
A negative correlation was observed between the stage and the one-year total radiation dose in MM, but it was not statistically significant.
Discussion
Radiation at high doses destroys tissues and has a short-term effect. Cancer and other long-term negative effects are also possible. Low-dose radiation, on the other hand, has been shown to be harmful in studies. The majority of estimates of low-dose radiation’s adverse consequences come from Japanese studies of atomic bomb survivors. Radiation exposures of 5 to 150 mSv, with a mean of about 40 mSv, resulted in a significant increase in cancer risk [12-14]. Even at cumulative doses as low as 5 to 50 mSv, in a large cohort study including over 400,000 nuclear radiation workers, a 1-2% dose-related increase in cancer deaths from chronic exposure to low levels of radiation was found [12,15]. This data supports the conclusion of Biological Effects of Ionizing Radiation (BEIR) VII, which found “a linear, no-threshold dose-response relationship between ionizing radiation exposure and cancer development in humans” [12,16].
In haematology, frequent medical imaging is routine, and new investigations have raised concerns about radiation dangers. Despite the development of machines that emit less radiation, patients are still exposed to radiation during diagnostic and follow-up examinations. In the USA, the NRCP (National Council on Radiation Protection and Measurements) annual occupational radiation exposure is 20 mSv/year (on an average of 5 years); no more than 50 mSv in any year and 100 mSv in 5 years [8]. In the current study, which focused on the radiation doses in the first year following diagnosis, the overall median radiation doses in that year were 36.2 for AML, 66.4 for DLBCL, and 26.0 for MM. It was shown that DLBCL patients in particular received high doses of radiation.
Patients with DBBL are exposed to radiation, particularly when undergoing CT and nuclear medicine (PET/CT and scintigraphy) exams. These examination indications appear to be used to stage the disease and assess the response to treatment. It is worth noting that, whereas the median dose for CT in DBBL is 35.0 (0-139), it is 26.5 (0-101) in AML. The primary indication for using CT in AML is to investigate the site of infection, such as neutropaenic fever.
In a study, the median cumulative radiation exposure after allogeneic stem cell transplantation (days 30-200) was determined as 92 mSv (range 1.2-300) for diagnostic radiological procedures [12]. This high figure is particularly concerning for patients who are expected to live longer, particularly children.
The CED supplies a generic estimate of the overall harm to the patient caused by the radiation exposure and allows for a rough comparison between different groups, but it provides only an approximate estimate of the true risk. To capture a more homogeneous patient group for the current investigation, a brief time frame (between 2016 and 2019) was discussed, taking into account the possibility that radiological instruments of various modernity could be used throughout time.
Regardless of the long-term or short-term effects, we believe it is critical to limit diagnostic radiation exposure in patients as much as possible. Many technical and technological features can be used to achieve this goal, such as reducing the severity and duration of exposure [9,10]. It should be regarded as one of the requirements of acting in accordance with the principle of causing the least harm to the patient first [11]. In this regard, every haematology clinic should make the required arrangements to provide patients with the necessary self-control in terms of radiation exposure.
There were a few limitations of this study. First, even though a power analysis was done with the support of the biostatistics department, this study had a retrospective design and only included a small number of patients. It is recommended that additional prospective trials are conducted including several patients. Second, when calculating CED, the lack of a weighting factor to verify the simulated organ absorbed doses was a limitation to the research.
Conclusions
The reference value of the radiation dose to which patients with haematological malignancies are exposed could not be discovered in the literature due to a lack of data. We believe that more research is needed on this subject. In this regard, our research can be considered a first publication.


