Introduction
Recently, technological progress, better availability, and higher quality of non-invasive neuroimaging is remarkable. Pathologies with smaller measurements can be investigated and treated earlier. Thanks to that we can extract very small intracranial aneurysms (VSIAs) from the group of intracranial aneurysms. It is crucial to diagnose aneurysms before they burst, to avoid or minimise neurological complications and death. Because unruptured intracranial aneurysms create a risk of subarachnoid haemorrhage (SAH), risk factors of rupturing such as sex, age, smoking, hypertension, aspect ratio, size, irregular shape, and location at bifurcation or posterior circulation should be considered [1].
The aim of our study was to analyse methods of treatment, their outcome, and complications in a group of patients with VSIAs.
Material and methods
For our study, we retrospectively reviewed collected data of all patients diagnosed with intracranial aneurysms in our centre in the years 2008-2014. In those years the number of patients, treated both invasively and conservatively, gradually increased up to 300 cases per year in 2014. During these years, coil embolisation was the main treatment method in our centre. In cases of wide-neck aneurysms stent-assisted coiling was always considered. Flow-diverters have not been used in our centre yet. Only the first procedure regarding particular aneurysms was taken into consideration during data analysis. Patients who did not undergo digital subtraction angiography (DSA) were excluded from this study. All patients who were not qualified to invasive treatment, including patients with VSIA, were also excluded. In total 444 patients met the inclusion criteria and were qualified to this study.
All aneurysms were visualised using 3-dimensional DSA (3D-DSA) on a Philips Integris Allura. Then, using 3D reconstruction, the aneurysm domes and necks were measured. The following parameters were obtained: width and length of the aneurysm neck; width, length, and height of the aneurysm dome. Bottleneck factors (BN) were counted in several combinations (Tables 1 and 2). The average size was calculated as the arithmetic average from all dome parameters.
Table 1
Calculations performed to obtain several options for bottleneck (BN) factors
Table 2
Comparison of bottleneck (BN) factors and average size among very small intracranial aneurysms (VSIAs) and the control group
| Mean size | VSIAs | Control group | p |
|---|---|---|---|
| BN minimal | 0.84 | 1.22 | 0.000001 |
| BN maximal | 1.39 | 1.91 | 0.000001 |
| BN width | 1.13 | 1.62 | 0.000001 |
| BN length | 1.01 | 1.47 | 0.000001 |
| BN average | 1.11 | 1.57 | 0.000001 |
| Average size | 3.26 | 7.06 | 0.000001 |
A cohort of 65 cases, representing 14.64% of the total population, met our criteria of VSIA, which were any measured dome dimension ≤ 3 mm. The group included 43 women and 22 men, with an average age of 53.28 (SD = 11.31) years. The remaining 379 cases (85.36%) were classified into a control group.
The analysed parameters were as follows: symptoms presented upon admission and their withdrawal after treatment, days spent in the Neurosurgery Department and Intensive Care Unit, and intraoperative complications. SAH was assessed using the Hunt and Hess scale and original Fisher scale [2-4].
For assessment of the immediate and long-term outcomes of endovascular procedures, residual patency rates on the three-item score of the Montreal scale (1 = total occlusion, 2 = neck remnant, and 3 = aneurysm remnant) were registered directly after embolisation and in follow-ups [5]. Neurosurgical treatment was assessed respectively based on completeness of clipping. Follow-up examinations were carried out at least six months after the procedure and employed either DSA (27 patients, 40.9%) or CT angiography (four patients, 6.1%). Upon patient discharge the Glasgow Outcome Scale (GOS) scores were assessed (1 – death, 2 – persistent vegetative state, 3 – severe disability, 4 – moderate disability, 5 – low or no disability) [6].
All statistical analysis was performed using Statistica 12 software. Due to non-normal distribution, all analysis was performed using Mann-Whitney U test, Kruskal-Wallis ANOVA, and McNemar’s test. IRB approval for this study, as well as for any retrospective study in our university, is not required by the Institution Ethical Committee.
Results
Among 65 VSIAs, 33 endovascular procedures (50.77%) were carried out, including 24 (72.73%) cases of unruptured and nine (27.27%) cases of ruptured aneurysms. Neurosurgical clipping was employed in 32 cases (49.23%), with 16 (50.00%) patients presenting SAH and 16 (50.00%) patients with no intracranial bleeding. Distribution of sex, age, and the presence of SAH in our study group corresponded to all analysed cerebral aneurysms.
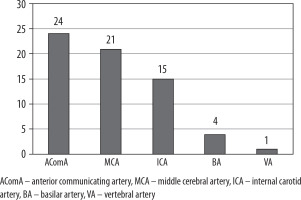
Predominant locations of aneurysms in our study group were anterior communicating artery (36.92%), followed by middle cerebral artery (32.31%) (Figure 1). Mean size is presented in Table 4.
Table 4
Efficacy of endovascular procedures using the Montreal Scale
| Montreal Scale score | Very small intracranial aneurysms | Control group |
|---|---|---|
| I | 23 (69.70%) | 176 (65.43%) |
| II | 3 (9.09 %) | 51 (18.96%) |
| III | 7 (21.21%) | 42 (15.61%) |
Upon admission to the hospital, SAH was presented in 25 (38.46%) patients with VSIA and 124 (32.72%) patients in the control group (p > 0.05).
Intraoperative complications were presented more often in patients with VSIA than in patients in the control group (16.92% vs. 13.19%) but without statistical significance (p = 0.42). Table 3 outlines noted complications. Considering each particular complication between groups, statistical significance was only discovered in brain oedema prevalence (p = 0.02). However, that result is probably caused by the existence of only one case. The most common complication in both groups was ischaemic stroke.
Table 3
Intraoperative complications
Stents were used in 17 cases (51.52%) of the very small aneurysm group. In the control group, they were used in 137 patients (50.74%).
Table 4 illustrates Montreal Scale results. In 25 cases, including procedures in the VSIA and control group, the Montreal Scale results were not assessed due to interruption of the procedure. In another two cases it was not possible to assess the Montreal score retrospectively. In 69.70% of embolisation procedures at VSIA complete obliteration was achieved. The average Montreal Scale result was 1.31 (SD = 0.66). Due to technical difficulties, intervention failed in four (12.12%) cases. Twice the failure was caused by drug-resistant vasospasm. In one case the coil evacuated from the aneurysm sack. In the last patient the coil could not be placed into the sac of the aneurysm despite numerous attempts. In the control group, average Montreal Scale score was 1.37 (SD = 0.64) and did not differ with VSIA group (p = 0.44). Of the 21 (7.78%) interrupted embolisations in the control group: three times the aneurysm ruptured, drug resistant vasospasm caused a failure eight times, and complications with insertion of coils into the aneurysm sac resulted in seven failed procedures. Information about the remaining three cases could not be retrospectively obtained.
All neurosurgical procedures in both groups of patients led to complete isolation from circulation.
The average hospitalisation period of patients in the VSIA group was 15 days, including 11 days in the Neurosurgery Department and four days in the Intensive Care Unit. In the control group the results were 10, eight, and two days, respectively. Differences were not statistically significant (p = 0.44, p = 0.52, and p = 0.36, respectively).
178 (58.75%) patients who underwent endovascular embolisation had arrived at a follow-up examination, including 21 patients (63.64%) with VSIA. Average results in the Montreal Scale differ between the VSIA (1.10, SD = 0.30) and control groups (1.38, SD = 0.67), but there is no statistical significance (p = 0.07).
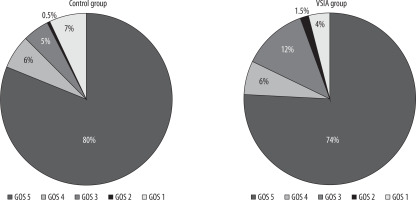
Mean Glasgow Outcome Scale scores were 4.45 (SD = 1.08) and 4.54 (SD = 1.12) for the VSIA and control groups, respectively, with no statistically significant difference (p = 0.24). As had been presented in Figure 2, the majority of patients recovered well after the procedure. There was nonsignificant (p = 0.45) dissimilarity in mortality. Thirty patients in our study died: three (4.62%) in the VSIA group and 27 (7.12%) in the control group.
Discussion
In the literature, very small intracranial aneurysms are defined as intracranial aneurysms with a diameter smaller than or equal to 3 mm [7-9]. Other sources qualify these aneurysms to a group called “baby aneurysms” [10,11]. Unfortunately, the sources do not indicate which dimension is analysed in qualification of the aneurysm into the VSIA group; therefore, in our study, to the VSIA group we qualified aneurysms with any dimension less than 3 mm. Based on our criteria we qualified 65 patients with VSIA for the study.
Analysing the therapeutic problems thast VSIA can cause, therapeutic decisions should be individual for each patient [12,13]. Keeping in mind patients’ health and considering the possible complications, we should choose the best treatment from the following invasive methods: neurosurgical clipping, endovascular coiling, or by-pass surgery [14-16]. In our centre two of those methods of treatment are being performed: surgery clipping and endovascular procedure. As by-pass surgery is not an available option, we cannot analyse the effectiveness of this method in aneurysm treatment. We realise that this is a restriction for our research. In the literature there are not many articles comparing endovascular and neurosurgery treatment for very small aneurysms [12,13]. Therefore, in our study, we decided to analyse treatment methods, complications, and mortality with regard to the control group of patients with larger aneurysms.
In patients with VSIA there are many factors that can cause technical and therapeutic problems. In view of their small size, the VSIA are a therapeutic problem for neurosurgeons and invasive radiologists. The small size of the aneurysm restricts the freedom of the operator’s movement, and it increases the risk of uncontrolled dislocation of the catheter and microaneurysm rupture. Additionally, in very small aneurysm there is greater risk of putting the microcatheter too close to the primordial site of the rupture [17-19]. In our centre, due to technical problems, 12.12% of treatment procedures have failed. In the literature there are also data about interrupted operations due to technical problems [13]. Other authors mention coil-related problems, but they do not specifically indicate what the problems are and how often they occur [20]. In the literature there is no definite position that would indicate the frequency of occurrence of technical problems during the procedure and what complications should be included in this group. Accordingly, we cannot clearly compare our results with other publications. However, we can compare these results between the VSIA group with the control group, where there is no statistical significance.
In our centre the most common complication was ischaemic stroke. It was also described as a complication of treatment in other centres, but it was not the most common [21,22]. Data show that frequent ischaemic events were related to patient age and other adverse factors. Other complications seen in our centre such as rupture of the aneurysm during surgery are also described in other publications. It was 1.54% in our centre, while in the literature it is described as 3.92% to 4.90% [10,20,23]. Some authors describe how VSIA rupture associated with procedure occurs twice or even five times more than in larger aneurysms [17,24,25]. On the other hand, in other sources the authors reported that during the procedure there were no complications [24,26].
In our study we checked bottleneck factors, and our analysis showed that VSIA have lower values of bottleneck factors then aneurysms in the control group. In connection, we could expect more complications in this group of patients than in the control group [22]. However, in our study the complication ratio was comparable in both groups. The time of hospitalisation was similar for patients with VSIA and patients with other intracranial aneurysms investigated in our study. Finally, there were no differences for these groups and expectedly the mortality was not higher in the group of patients with VSIA. Our mortality outcomes are comparable with data in the literature [27].
Conclusions
In conclusion, the criteria used in this study to isolate patients with VSIA from patients with other intracranial aneurysms may help those patients who need more precautions in the therapeutic process. In addition, the development of techniques used in the treatment of aneurysms allows the safe treatment of minor changes by both endovascular and surgical methods. It appears that these aneurysms can be treated as effectively and safely as larger aneurysms. This topic requires further investigation.