Introduction
The hepatobiliary system includes “hepato”, which refers to the liver, and “biliary”, which refers to the gall bladder and bile ducts. The liver is a highly vascular organ that performs the complex tasks of both synthesis and detoxification. Thus, the liver is fertile ground for harbouring infections and infestations. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and the most common cause of death in patients with cirrhosis. Gall bladder cancer is the most common malignancy of the biliary tract and the third most common cancer in the gastrointestinal tract [1]. Abdominal ultrasonography (USG) is a baseline modality for evaluating hepatobiliary lesions. Moreover, abdominal USG is beneficial for the follow-up examination of primary lesions or during surveillance in chronic liver diseases. Focal hepatobiliary lesions may be incidentally detected on b-mode USG and further examined through colour Doppler. Indeterminate lesions on conventional ultrasound can be evaluated through abdominal contrast-enhanced ultrasound (CEUS) [2]. Ultrasound contrast agents currently available in India act as pure blood pool agents that do not extravasate into the interstitial fluid [3]. This leads to intense enhancement of the vascular system, allowing superior depiction of vascular morphology on CEUS compared with either computed tomography (CT) or magnetic resonance imaging (MRI). CEUS is the least invasive contrast imaging technique, and it can be used with portable machines and interventional assistance. Moreover, CEUS is a non-nephrotoxic and cost-effective procedure that can be used in children [4-6]. However, unlike contrast-enhanced CT (CECT) and contrast-enhanced MRI (CEMRI), CEUS has limited spatial resolution, cannot provide a 3-dimensional image, and has limitations in evaluating obese patients with hepatic steatosis and subdiaphragmatic lesions [7,8] In this prospective study, we investigated the efficacy of CEUS in diagnosing hepatobiliary lesions and correlated the ultrasound diagnosis with pathology findings.
Material and methods
This prospective study was performed between August 2018 and August 2020 and included 50 patients with hepatobiliary lesions, who each gave their written informed consent. The inclusion criteria were the presence of focal lesions on the baseline ultrasound scan of patients with cirrhosis, suspected liver metastasis in a known case of primary malignancy, and incidental detection of focal liver lesions (FLLs) on sonography. We excluded patients with a history of contrast allergy or those who were pregnant or breastfeeding.
Baseline greyscale and Doppler ultrasonography was performed in all patients. Furthermore, contrast-enhanced imaging was conducted using an Affiniti G70 ultrasound scanner (Philips Healthcare). Routine imaging was performed using a convex probe (transmitting frequency, 5.0 MHz; mechanical index, 0.1), and the focus was set on the region of interest. Using a timer, we recorded video clips in DICOM format after administering the ultrasound contrast. Patients with hepatobiliary lesions, aged between 18 and 70 years, were included.
All patients were administered sulphur hexafluoride as the intravenous contrast medium. Sulphur hexafluoride (SonoVue, Bracco Imaging SpA, Milan) is available as a lyophilized powder in vials, which is reconstituted with 5 ml of saline. The reconstituted solution comprised 8 ml of sulphur hexafluoride in microbubbles. Each patient received 2.4 ml of this solution, followed by a 10-ml saline flush.
Ultrasound imaging was performed before and after the intravenous administration of sulphur hexafluoride in regular and contrast modes. Real-time postcontrast imaging was performed during arterial, portal venous, and late phases. The arterial phase was acquired at 10-20 seconds of contrast administration, the portal venous phase (PVP) was acquired at 30-45 seconds, and the late phase was taken after 120 seconds of contrast injection. If multiple space-occupying lesions were noted, the largest lesion was evaluated. Relevant frozen images and cine loops were recorded. The contrast enhancement pattern was classified into no vascularity, peripheral enhancement, central enhancement, and homogenous/heterogenous enhancement of the whole lesion; washout of contrast from the lesion; and the persistent enhancement of the lesion in the delayed phase. Patients were monitored for 30 min after the completion of imaging. A provisional diagnosis was established on the basis of non-enhanced and contrast images.
Analyses of collected data
Subjectively, all the contrast phases were examined, and the perfusion pattern of the lesion was compared with the liver parenchyma around it. We studied the contrast arrival time, pattern of enhancement, vessel distribution, degree of enhancement, and wash-out timing. Plotting time intensity curves (TIC) allowed for the acquisition of objective information. Time to peak intensity, peak intensity, area under the curve, and wash-out time are some of the metrics that were investigated from TICs. By comparing the above-cited metrics of the lesions with those of the normal liver parenchyma around them, significant information was obtained.
In general, persistent enhancement in the portal venous and late phases allowed for the identification of the majority of benign lesions. In the delayed phase, benign lesions showed hyper-enhancement or iso-enhancement compared to the surrounding liver parenchyma, while malignant lesions had a hypo-enhancing appearance. The arterial phase was particularly helpful for benign lesions such as haemangiomas because the later showed typical enhancement features, i.e. peripheral nodular enhancement with centripetal filling. Regardless of the arterial enhancement pattern, early washout in the portal and late phases was the defining feature of the majority of hepatic malignancies on CEUS.
Radiological diagnosis was established on the basis of CEUS findings, and lesions were further categorized as benign and malignant. FNAC was done initially for the pathological diagnosis because it is minimally invasive. A biopsy was performed for the definitive diagnosis in cases of inconclusive aspirations, when smears had a few cells but not enough for a conclusive diagnosis, and in cases of insufficient aspirations. Pathological diagnosis was considered as the gold standard. The data were entered into Microsoft Excel spreadsheets, and the results were statistically evaluated using SSPS version 6.0 for Windows (SSPS Inc., Chicago, IL, USA). The correlation of CEUS findings with pathological findings was evaluated, and the sensitivity, and malignant lesions were calculated.
Results
The present study recruited 50 patients (29 women and 21 men) over a period of 2 years. CEUS findings were correlated with pathological findings. The ages of the patients ranged from 18 to 70 years, with most of the patients (n = 19, 38.2%) belonging to the age group 51-60 years, followed by the age groups 60-70 years (n = 15, 30%) and 41-50 years (n = 10, 20%). A total of 28 (56%) patients had a single lesion, whereas 22 (44%) patients had multiple lesions. On the baseline ultrasound scan, chronic liver disease and ascites were the most common presentations. Furthermore, the most common lab findings were deranged liver function tests and increased a-fetoprotein levels; these findings were noted in 14.7% of the patients.
On CEUS, all 50 patients showed enhancement on arterial phase imaging, whereas 5 lesions exhibited persistent enhancement in portovenous and delayed phases of imaging. On the basis of CEUS findings, a diagnosis of benign lesions was made in 5 (10%) patients and malignant lesions in 45 (90%) patients. The correlation of radiological findings with pathological findings was examined in all patients. On pathological examination, 46 (92%) and 4 (8%) patients were observed to have malignant and benign lesions, respectively. A discrepancy was noted in the assessment of one lesion, which was believed to be haemangioma on the basis of CEUS findings but was found to be metastasis on histopathological examination.
Among 45 (90%) patients with malignant lesions, metastasis was the most common finding (n = 20, 40%), followed by HCC (n = 17, 34%), GB carcinoma (n = 7, 14%), lymphoma (n = 1, 2%), and cholangiocarcinoma (n = 1, 2%) (Figures 1-3). Most of the metastases appeared as hypoechoic nodules in contrast to the enhanced background of the normal liver parenchyma. The reason for the high prevalence of metastasis in this study is the high prevalence of cancer in this region.
Figure 1
50-year-old male, a known case of colonic adenocarcinoma presented with pain right hypochondrium for the last one month. A) B-mode conventional ultrasound image shows hyperechoic mass in liver (arrow). B-C) Ultrasound images obtained after contrast medium administration show nodules which are to be hypoechoic (arrows) in comparison with surrounding liver parenchyma in portal and late phases – hypovascular metastasis. D) Histopathology confirmed the diagnosis of liver metastasis
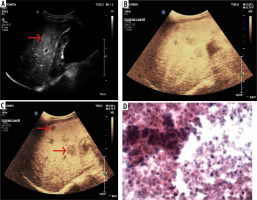
Figure 2
55-year-male, a known case of hepatitis-C presented with history of weight loss. A) B-mode conventional ultrasound image shows hypoechoic mass in left lobe (arrow) of liver. B-C) Ultrasound images obtained after contrast medium administration shows an eccentric nodule to be hypervascular (arrow) in arterial phase (B) and portal phase (C) with washout in delayed phases that was suggestive of hepatocellular carcinoma. D) The diagnosis was confirmed by histopathology
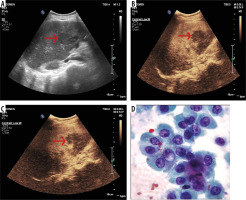
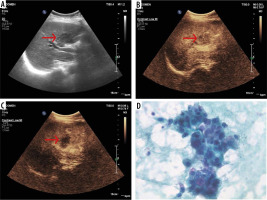
Figure 3
60-year-old female with history of weight loss and heaviness in right hypochondrium. A) Baseline ultrasound illustrates an ill-defined heterogeneous mass (arrow) at the porta hepatis. B) Contrast-enhanced ultrasound (CEUS) after 25 s after microbubble contrast administration demonstrates mild enhancement (arrow). C) CEUS at 80 s after microbubble contrast administration demonstrates hypoenhancement (arrow) within the lesion. D) On histopathological correlation, diagnosis of intrahepatic cholagiocarcinoma was confirmed
The sensitivity, specificity, PPV, and NPV of CEUS for the detection and characterization of hepatobiliary lesions were 100.00% (95% confidence interval [CI]: 39.76-100.00%), 97.83% (95% CI: 88.47-99.94%), 80%, and 100.00%, respectively. Hepatobiliary lesions were diagnosed with an accuracy of 98.00% (95% CI: 89.35-99.95%) on the basis of CEUS findings (Table 1). No side effects of ultrasound contrast were noted in any patient.
Table 1
Correlation of contrast ultrasound findings with pathological diagnosis (gold standard). Sensitivity, specificity, positive predictive value, and negative predictive valueof contrast-enhanced ultrasound for the detection and characterization of hepatobiliary lesions are depicted
Discussion
This study recruited a total of 50 patients. On the basis of CEUS findings, 45 lesions were diagnosed as malignant and 5 as benign. The diagnosis of all the lesions was correlated with pathological findings. On pathological examination, 46 and 4 patients were determined to have malignant and benign tumours, respectively. Among the 45 patients with malignant tumours, metastasis was the most common finding (n = 20, 40%), followed by HCC (n = 17, 34%), GB carcinoma (n = 7, 14%), lymphoma (n = 1, 2%), and cholangiocarcinoma (n = 1, 2%). Hafeez et al. reported that metastatic lesions were observed in 37.5% of their entire sample [9]. However, Goel et al. [10] observed 42.1% of metastatic lesions in their study. In the present study, 17 (34%) patients were found to have metastatic lesions. A reason for the high number of metastatic lesions in the present study might be the high prevalence of cancer in this region.
Washout in portovenous and delayed images is the most crucial criterion to differentiate malignant lesions from benign lesions [11]. Washout is defined as the decline in the enhancement of a lesion to a level that is less than that of the adjacent liver parenchyma after the peak arterial phase enhancement. Lesion washout is influenced by 2 criteria: the haemodynamics of the liver and the vascular features of the lesion [12]. In our study on contrast scans, all lesions showed enhancement in the early arterial phase. Of the 5 lesions showing persistent enhancement in portovenous and delayed phases, 2 were diagnosed as haemangiomas and one as HCC.
The most crucial characteristic features of haemangiomas on CEUS are peripheral nodular contrast enhancement and centripetal fill-in. Hyperenhancement is sustained through the late phase in contrast to its crucial differential diagnosis of haemangioendotheliomas and other malignant mesenchymal liver tumours [12,13].
In our study, one case of cholangiocarcinoma on CEUS showed enhancement in the arterial phase followed by washout in the portovenous phase. In most of the cholangiocarcinomas, irregular rim-like hyperenhancement in the periphery of the tumour was noted during the arterial phase; this lasted until the portal phase in the majority of cholangiocarcinomas [14,15].
After metastasis, HCC was the most common lesion found in our study. Of the 17 cases, 16 showed arterial enhancement followed by washout, and one demonstrated progressive enhancement on portovenous and delayed phase images. Thus, the diagnosis of benign (haemangioma) lesions was established on the basis of CEUS findings. On pathology, HCC was diagnosed. HCC is characterized by arterial phase hypervascularity and later washout on CEUS. Arterial phase enhancement is often diffuse or heterogeneous. Peripheral rim-like enhancement is unusual in HCC and commonly observed in metastases or intrahepatic cholangiocellular carcinoma (ICC) [16]. Large HCCs often exhibit non-enhancing areas due to necrosis or internal haemorrhage. Washout in HCC tends to be late and often begins later than 90 s after the injection of the contrast medium, whereas metastases or ICCs consistently show rapid washout (< 60 seconds) [17]. Most small cholangiocarcinomas, which are infrequently detected during HCC surveillance, can be differentiated from HCC because of the presence of rim-like arterial enhancement and rapid washout. Washout is slower or may even not be observed in the occasional cases of well-differentiated HCC. Washout timing is related to the pathological differentiation of HCC.
Well-differentiated HCC tends to show later washout or no washout, whereas poorly differentiated HCC tends to show rapid washout [18,19].
In our study, one lesion was found to be lymphoma on pathology. The lesion exhibited arterial hyperenhancement followed by washout in the portal phase. On CEUS, hepatic lymphoma typically exhibits rapid arterial enhancement, followed by hypo-enhancement in the portal and late phases [20,21]. In a previous study, lymphoma lesions exhibited hypo-enhancement during the late phase [22].
In our study, 7 patients received a diagnosis of GB carcinoma, and this diagnosis correlated with pathological findings. CEUS is useful for differentiating hyperechoic sludge from malignant lesions. Because sludge has no blood supply inside it, it exhibits complete non-enhancement on both arterial and venous phases. The diagnostic accuracy was 100% in our study, and the result is similar to those of some previous studies [23,24].
The present study showed comparable sensitivity, speci-ficity, and efficacy in evaluating hepatobiliary lesions as compared to the studies done by Soye et al. [25], Strobel et al. [26], and Wu et al. [27], Wu et al. [28], and Wang et al. [29]. A recent study done by Von Herbay et al. found that, compared with baseline US, CEUS after Levovist injection improved the sensitivity for the discrimination of malignant versus benign liver lesions from 85% to 100%, and the specificity from 30% to 63%. All lesions that had homogeneous enhancement in the late phase of Levovist enhancement were benign. As compared to this study, in the present study the sensitivity and specificity were 100% and 96.8%, respectively. In our study all benign lesions also had homogeneous enhancement in the late phases.
CEUS has some limitations. Unlike CECT and CEMRI, which can explore the entire liver in depth, CEUS cannot provide a 3-dimensional view. Sensitivity and specificity are decreased in patients with fatty liver disease and obesity. Furthermore, unlike cross-sectional imaging techniques, CEUS does not permit an assessment of multiple focal liver lesions at the same time. Deep-seated and subdiaphragmatic lesions may not be accessible, and extremely small lesions may be missed. False-positive cases have been associated with scars, fibrosis, and necrotic lesions [30]. Finally, CEUS is more expensive than CECT in some countries; therefore, CEUS use is usually limited to expert centres and should be performed by skilled operators to prevent nonconclusive or erroneous examinations [26]. Artefacts can be encountered occasionally, including shadowing and prolonged liver enhancement [31-33], which are characterized by late and diffuse heterogeneous enhancement of the liver after UCA administration. Knowledge of these artifacts is crucial to prevent misinterpretations that can lead to a false-positive diagnosis. However, none of our cases exhibited prolonged liver enhancement.
None of the patients included in this study exhibited any side effects after the administration of the ultrasound contrast; this finding is in agreement with that of a previous study reporting that ultrasound contrast agents are generally safe and without any major side effect [34].
With the help of an adaptive algorithm, microvascular imaging (MI) innovative ultrasonic technology shows microvascular flow in the tissues similar to contrast ultrasound (CEUS). MI has been employed in several earlier investigations of the differentiation of liver lesions. A study by Yang et al. [35] established a superiority in the identification of microvascular blood flow signals with MI in HCCs as compared to colour Doppler flow imaging (CDFI). Dubinsky et al. [36] found that MI showed more central and peripheral arteries in relation to liver lesions, as compared to CFDI and power Doppler (PD) imaging, and hence helped to better distinguish between malignant and benign liver neoplasms. When comparing quantitative MI to quantitative CEUS, there are various benefits.
For example, in MI, no contrast medium is needed. Additionally, CEUS imaging has a time restriction due to the gradual disintegration of the phospholipid microbubbles, whereas MI’s informative value is unaffected by time. In a recent polit study done by Kratzer et al. [37] the authors showed a substantial correlation between the CEUS and MI results in cases with liver metastasis. However, we feel that larger studies are required to make a definite comparison between these 2 techniques.


