Introduction
Transthoracic ultrasound (US) has long been used suboptimally for thorax assessment, possibly because of the sternum, overlying rib cage, and aerated lungs. Transthoracic US examination of the pleura and pleural space is a highly specific and sensitive tool for the assessment of pleura and pleural space pathologies. Sonographic characteristics might also help to discriminate between underlying benign and malignant pathologies. Transthoracic US examination provides real-time evaluation of the pleura and pleural space. It is free of radiation hazard and requires only a short time for examination [1].
Radiological assessment used commonly for the detection of pleural pathologies includes chest X-ray (CXR), US, and computed tomography (CT). The sensitivity and specificity of CXR are of limited value for detecting small pleural effusions because it cannot reliably differentiate between small pleural effusion and pleural thickening. Chest CT is a highly sensitive and specific investigation for diagnosing pleural malignancies. However, the drawbacks of CT include high cost, ionising radiation exposure, and availability only in tertiary care centres [2]. Transthoracic US, in comparison to CXR and CT, has been found to be superior in diagnosing many lung pathologies and pleural effusion [3-6]. Pleural effusion appears as an echo-free space between visceral and parietal pleura. Transthoracic US is far more sensitive and specific to detect small pleural effusion (< 5 ml) and can even differentiate small pleural effusion from pleural thickening. It can detect physiological amounts of pleural fluid and is 100% sensitive for effusion > 100 ml [7,8]. It can quantify the pleural effusion and can characterise the type of fluid and hence the pathology based on echogenicity [2,3]. Quantification of pleural effusion can be done with the Goecke formula:
where EV is the pleural effusion volume, X is the craniocaudal extent of pleural effusion at the dorsolateral chest wall measured in an erect/supine position with probe oriented longitudinally, LDD is the lung base to mid-diaphragm distance/sub-pulmonary height of effusion in cm, and 70 is an empirical factor or constant [9]. Difficulty arises in differentiating between minimal pleural effusion and pleural thickening because both can appear anechoic on greyscale USG, so the echo-free area does not guarantee the presence of pleural effusion [10,11]. Colour Doppler detects any movement of body fluid and displays it as coloured images. A colour signal may appear within the fluid collection in the pleural space during the respiratory and cardiac cycle, known as “fluid colour sign”. In the case of pleural thickening, no colour is detected because pleural thickening has no movable part [10-12].
The use of a high-frequency (7-12 MHz) linear array probe allows superior resolution for refined assessment of the pleura [13-15]. The pleura appears as an echogenic line on USG [16]. Transthoracic US is most sensitive for the detection of the pleural septations. Pleural adhesions are seen as free-floating septa in the pleural effusion, which traverse from parietal pleura to visceral pleura. However, the definite diagnosis of pleural pathologies is made by histopathological examination (HPE). Pleural thickening and fibrosis can resemble pleural effusion on CXR and even on CT chest, resulting in an unnecessary trial of needle aspiration. Many studies have shown that there were no adhesions if the pleural excursion was greater than 2 cm in lower thoracic wall and greater than 1 cm in upper thoracic wall [13,17]. US assessment of the pleura before thoracoscopy assists in choosing the perfect site for thoracoscopy, thus preventing major injury to the lungs. Ascertaining the presence and location of pleural adhesions by US can also aid in deciding the trocar insertion site during thoracoscopy [18].
Medical thoracoscopy is commonly used for various diagnostic and therapeutic procedures. It is carried out for complete exploration of the hemithorax for pleural adhesions and pleural thickening, and it documents other adhesions not previously identified [18-20]. It can be performed under local anaesthesia and is considered a gold standard for managing pleural adhesions present between the visceral and parietal pleura and for taking pleural biopsies [18,21]. Pleural adhesions prevent the lung from collapsing at the start of thoracoscopy and thus increases the risk of lung injury from video telescope. In severe cases, it can prevent the access to the pleural space, requiring conversion to open thoracotomy [22-24].
The purpose of this study was to evaluate the use of the transthoracic US for pleural adhesions and pleural thickening and to quantify pleural effusion by standardised sonographic techniques and its comparison with thoracoscopic findings. An attempt was also made to assess the association between pleural thickness and pleural nodularity with pleural histopathological examination findings.
Material and methods
The current study was conducted in a tertiary care institute for a period of one and a half years, from March 2021 to September 2022, after approval from the Ethical Committee. All patients who presented with suspected/undiagnosed pleural pathologies and further undergoing evaluation with medical thoracoscopy in addition to routine blood and chest X-ray investigation were included in this study after taking their informed consent. However, patients who presented with pneumothorax, previous pleurodesis, and were markedly unstable were excluded from the examination.
USG procedure
USG of pleura and pleural space was done with linear (7-11 MHz) and curved (2-5 MHz) US probes within one week prior to medical thoracoscopy. All patients were examined in a sitting position with the arm on the examination side elevated and the hand positioned behind the neck, and they were asked to breathe deeply, exaggerating the rate of respiration. The US probe was applied tangentially to the chest wall. In the intercostal spaces, the probe was held at a 45o angle relative to the longitudinal direction in the acoustic window, and focus was set on the pleural line. The USG probe was applied, and the nature of echogenicity of pleural effusion was assessed. USG was also performed to look for the presence or absence of gliding sign, which was seen as bright to and fro movement visible at the pleural line during respiratory movements, which is due to normal gliding of the visceral and parietal pleura over each other. The presence of gliding sign indicated the absence of adhesions. A distinct focal point in the image of the viscera was observed as the visceral pleura moved during respiration. The distance of the longitudinal excursion of the selected point was measured in relation to the chest wall. If the sliding of the selected focal point of visceral pleura was less than 2 cm in the lower thoracic wall and less than 1 cm in the upper thoracic wall, it was taken as suggestive of adhesions. The colour fluid sign was also evaluated, which indicated pleural effusion. Pleural thickening was measured, and pleural nodularity along the visceral and parietal pleura was assessed.
Medical thoracoscopy
Thoracoscopy was done by an experienced pulmonary medicine specialist after US, who was blinded to the US finding results. For thoracoscopy, the patient lay on their healthy side in a lateral decubitus position with the involved side up. The midaxillary point at the level of fourth or fifth midaxillary intercostal space allowed the best visualisation of the thoracic cavity. Other points of entry were used on the basis of the US guidance findings for easy access of the pleural cavity. After standard surgical scrub techniques for both physician and patient, a vertical incision was made with the scalpel through the skin and subcutaneous tissue at the selected point of entry, appropriate to the size of the trocar used. The incision was parallel and in the middle of the selected intercostal space. The trocar was then inserted in a corkscrew motion until the sudden release of resistance was felt, suggesting the trocar was in the pleural cavity. The trocar was then removed, and the cannula was placed 1-3 cm within the pleural cavity and was held in position by the assistant. The thoracoscope was placed in the cannula and advanced into the pleural cavity under direct vision through the trocar. If necessary, the pleural fluid was removed with a suction catheter or directly through the working channel of the thoracoscope. The pleural space was inspected via thoracoscope, either directly or indirectly by video for the pleural fluid, pleural adhesions/septations, and pleural thickening. A pleural biopsy was taken and sent for the HPE. Data were collected regarding the pleural fluid, presence and location of pleural adhesions, and pleural thickening on thoracoscopy and compared to find out the sensitivity and specificity of the transthoracic US for detecting pleural adhesions, pleural thickening, and amount of pleural effusion. Data were also compared with the histopathological examination findings.
Statistical analysis
The presentation of the categorical variables was done in the form of number and percentage (%). The quantitative data were presented as the means ± SD and as median with 25th and 75th percentiles (interquartile range). The comparison of the variables that were quantitative in nature was performed using the independent t-test. The paired t-test was used for comparison of the amount of PLEF on USG and thoracoscopy. The comparison of the variables that were qualitative in nature was performed using the c2 test. If any cell had an expected value of less than 5, Fisher’s exact test was used. Univariate linear regression was used to estimate the amount of pleural effusion (PLEF) measured on thoracoscopy based on the amount measured on ultrasound. Sensitivity, specificity, positive predictive value, and negative predictive value of the gliding sign was calculated, and a USG was performed to detect pleural septations. The data entry was done in Microsoft EXCEL, and the final analysis was performed with the use of statistical analysis software. For statistical significance, a p-value of less than 0.05 was considered statistically significant.
Results
The sonographic findings were compared with medical thoracoscopy and histopathology. A total of 31 patients were included in the present study. The maximum number of patients were in the age group of 51-60 years (32.26%), with the mean age of patients being 59.48 ± 11.8 years. Most of the patients in the study were male (58%), and 18 patients (58.06%) had pathology on the right side. In our study, pleural effusion was calculated using the Goecke formula in the erect position, which yielded a mean pleural effusion of 2008.84 ± 770.90 ml (mean ± SD) on USG. The amount of pleural effusion drained during medical thoracoscopy was 1998.39 ± 1126.53 ml (mean ± SD).
Echogenic pleural effusion was present in 12 (38.7%) patients, of whom 11 had haemorrhagic pleural effusion drained during medical thoracoscopy. Among patients with anechoic pleural effusion, 3 (15.79%) had haemorrhagic fluid on thoracoscopy. Out of 19 patients with massive pleural effusion (> 1500 ml), 11 (57.89%) had malignancy and 8 (42.11%) had benign pathology. Pleural thickness of more than 5 mm was observed in 14 patients (45.16%). These patients had multiple nodules on thoracoscopy, with 11 (78.57%) of them having a malignant cause. Pleural thickness of less than 5 mm was seen in 17 patients, of whom 16 (94.1%) had multiple nodules and only 1 (5.9%) had sparse nodules. Malignant aetiology was confirmed in 4 patients (23.5%).
Septations were present in 18 patients and absent in 13, as confirmed on thoracoscopy. Of these, 10 patients (32.26%) had thick septations (> 5 mm), with 4 such patients (40%) having malignant aetiology. Eight patients (25.81%) had septations less than 5 mm, and 3 of them (37.5%) had malignancy. Septations were absent in 13 patients (41.94%); however, malignancy was seen in 8 of these patients (65.54%). The gliding sign was present in 4 patients (12.9%) and absent in 8 patients (25.81%), while in the rest of the patients it could not be assessed due to large pleural effusion. 8/12 (66.6%) cases showing an absent gliding sign on transthoracic USG had septations on thoracoscopy. However, one patient (25%) with a positive gliding sign had septation detected on thoracoscopy. The colour sign was present in all patients. Diaphragmatic nodules were present in 9 patients (29%), all of whom had malignant pathology. In cases where diaphragmatic nodules were absent, malignant pathology was present in 6 patients (19.35%). Biopsy was performed in all cases with suspicious aetiologies.
Benign pathologies were reported in 16 patients (51.61%) and malignant pathologies in 15 patients (48.39%). Granulomatous lesions (25.8%) were the most common benign pathology, and metastasis (29%) was the most common malignant pathology. Metastatic adenocarcinoma was the most common metastatic variant in both males and females (6 out of 9 patients). Among the primary malignant pathologies, squamous cell carcinoma, poorly differentiated carcinoma, and clear cell papillary epithelioid carcinoma were the most common histological types. Figure 1-5 depict the ultrasound and thoracoscopic images of the corresponding cases of malignant and benign pathologies.
Figure 1
Case 1. A 77-year-old male with right-sided chest pain and cough. A) Chest X-ray shows right pleural effusion. B) Transthoracic ultrasound revealed echogenic pleural effusion with maximum pleural thickness along the VIII intercostal space at the paravertebral region with multiple costal and diaphragmatic nodules. C, D) Medical thoracoscopy revealed haemorrhagic pleural effusion with glistening white nodules along pleura. Biopsy confirmed poorly differentiated squamous cell carcinoma
Figure 2
Case 2. A 78-year-old male patient with right-sided chest pain and right-sided pleural effusion. A) Transthoracic ultrasound revealed echogenic pleural effusion. B, C) Maximum pleural thickness along the VII intercostal space posterior axillary line with few costal pleura nodules. D) Presence of multiple thin and thick septations. E, F) Medical thoracoscopy showed haemorrhagic pleural effusion with glistening white nodules along pleura. Thin and thick internal septations. Biopsy was suggestive of epithelioid mesothelioma with prominent papillary pattern
Figure 3
Case 3. A 61-year-old male with cough and right chest pain. A) Chest X-ray shows right pleural effusion. B) Echogenic right pleural effusion. C) On transthoracic ultrasound, maximum pleural thickness along the VII intercostal space posterior axillary line measuring 1 mm. D) Medical thoracoscopy revealed haemorrhagic pleural effusion. Necrotising granuloma on pleural biopsy
Figure 4
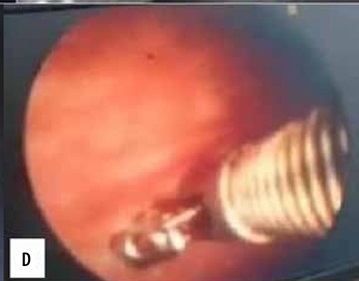
Case 4. A 41-year-old male patient with right-sided chest pain and cough. A, B) Transthoracic ultrasound revealed anechoic pleural effusion with 2700 ml of pleural effusion. C) Maximum pleural thickness along the VIII intercostal space paravertebral region measuring 2.3 mm with no diaphragmatic nodules. D) Medical thoracoscopy revealed straw coloured pleural effusion with few glistening white nodules along pleura. Total of 2500 ml of pleural effusion drained. Biopsy from nodule was suggestive of necrotising granuloma
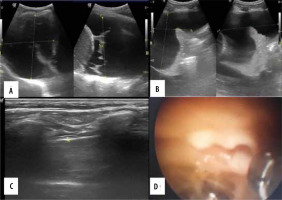
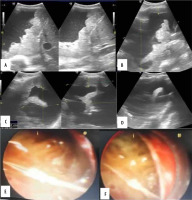
Figure 5
Case 5. A 57-year-old male patient, known case of renal cell carcinoma presented with right sided chest pain. A, B) Transthoracic ultrasound revealed echogenic pleural effusion with thickened irregular diaphragmatic pleura. C, D) Irregularly thickened pleura with maximum pleural thickness along the VIII intercostal space posterior axillary line measured 1.7 cm. Presence of thick septations. E, F) Medical thoracoscopy showed haemorrhagic pleural effusion with irregularly thickened pleura and multiple nodule. Thin and thick internal septations. Pleural biopsy revealed metastatic renal cell carcinoma
Discussion
Transthoracic USG examination is usually the first investigation for assessing pleural effusion and is highly sensitive and specific for evaluating pleura and pleural space pathologies. It also provides additional information such as the type of pleural fluid, pleural thickening, nodularity, pleural septation, etc. This examination allows real-time evaluation of the pleura and pleural space. Medical thoracoscopy is commonly used for various diagnostic and therapeutic procedures for managing pleural and chest pathologies.
The mean age in the present study was comparable to the study conducted by Cassanelli et al. [25], where the mean age was 63.4 ± 13.4 years, with male patients (69.01%) more commonly affected. In another study by Sasaki et al. [22], more patients were examined for pathologies on the right side (55.81%), which was also observed in the present study.
The univariate linear regression equation: 648.179 +1.317 × amount of PLEF (ml), was derived based on ultrasound findings. It yielded a p-value of < 0.001, a regression coefficient of 81.27%, and a Pearson correlation coefficient of 0.90, indicating a strong and statistically significant relationship. There was high accuracy of USG for quantification of pleural effusion, comparable to the study conducted by Ibitoye et al. [9], which yielded a Pearson coefficient of 0.87 using the Goecke formula in the erect position. In the present study, no significant association was found between the amount of pleural effusion and histopathological diagnosis of benign and malignant pathologies, with a p-value of 0.135. These findings were comparable to those of Qureshi et al. [4], who reported a combined p-value of 0.48 for the amount of pleural effusion and pleural malignancy.
Table 1
Transthoracic ultrasound greyscale findings
Table 2
Thoracoscopic findings
Table 3
Association of pleural thickening, irregularity, pleural septations findings (on ultrasound) with histopathological findings
| USG parameters | Benign (n = 15) | Malignant (n = 16) | Total | p-value | |
|---|---|---|---|---|---|
| Pleural thickening | Present (< 5 mm) | 13 (76.5%) | 4 (23.5%) | 17 | 0.0038* |
| Present (> 5 mm) | 3 (21.5%) | 11 (78.5%) | 14 | ||
| Total | 16 (51.6%) | 15 (48.4%) | 31 | ||
| Pleural nodularity | Nodular | 16 (51.6%) | 15 (48.4%) | 31 | NA |
| Septations | Absent | 5 (38.5%) | 8 (61.5%) | 13 | 0.477* |
| Thin septations (< 5 mm) | 5 (62.5%) | 3 (37.5%) | 8 | ||
| Thick septations (> 5 mm) | 6 (60.0%) | 4 (40.0%) | 10 | ||
Table 4
Histopathological findings of diaphragmatic pleura nodules
| Diaphragmatic pleura nodules | Pleural effusion on thoracoscopy | |||
|---|---|---|---|---|
| Benign | Malignant | Total | p-value | |
| Absent | 16 | 6 | 22 | < 0.05* |
| Present | 0 | 9 | 9 | |
| Total | 16 | 15 | 31 | |
Table 5
Univariate linear regression to estimate the amount of pleural effusion(ml) on thoracoscopy from amount of PLEF (ml) on ultrasound
| Variable | β coefficient | Standard error | p-value | Lower bound (95%) | Upper bound (95%) | Equation | R² |
|---|---|---|---|---|---|---|---|
| Amount of PLEF (ml) on USG | 1.317 | 0.117 | < 0.0001 | 1.077 | 1.558 | –648.179 +1.317* Amount of PLEF (ml) on ultrasound | 81.27 |
Table 6
Histopathological reports of study subjects
A significant association was observed between the echogenicity of pleural effusion on USG and haemorrhagic fluid drained during medical thoracoscopy (p < 0.0001), although no significant association was found between pleural fluid echogenicity and malignant pathology on histopathology. In a study by Yang et al. [26], homogeneous echogenic effusion was seen in haemorrhagic and empyema cases, and 8 out of 10 pleural malignancies showed homogeneous echogenic effusion. However, our study did not align with this finding because we also included patients showing echogenic foci in pleural effusion, whereas their study considered only homogeneous echogenic fluid.
In the present study, USG had a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 100%, 100%, 100%, and 100%, respectively, for detecting pleural septations (p < 0.001). This suggests that transthoracic USG is the most accurate diagnostic modality for detecting pleural septations in patients with pleural effusion. There was no significant association between the thickness of pleural septation and pleural malignancy in our study (p = 0.477). The results were comparable to a study by Sajadieh et al. [27], where only 2 out of 26 pleural malignancies showed pleural septations. Similarly, Qureshi et al. [4] concluded that there is no significant association between pleural septation and histopathologically confirmed malignant lesions (p = 1).
Transthoracic USG plays an important role in selecting patients for medical thoracoscopy. Extensive pleural septations were detected in 9 patients during pre-procedural transthoracic ultrasonography, who were subsequently rejected for medical thoracoscopy based on the USG findings. In our study, the sensitivity, specificity, PPV, and NPV of the gliding sign to detect pleural septations were 88.89%, 100%, 100%, and 75%, respectively, with a diagnostic accuracy of 91.67%. Thus, a significant association was observed between the gliding sign and septations, with a p-value of 0.007. This suggests that the gliding sign can accurately predict pleural adhesion/septations in the absence of pleural effusion. Similar suggestions were made by Cassanelli et al. [25], who reported sensitivity, specificity, PPV, and NPV of the gliding sign as 80.6%, 96.1%, 73.2%, and 97.4%, respectively. Our study also showed comparable results to the study by Sasaki et al. [22], which had sensitivity and specificity of 72% and 71%, respectively.
Pleural thickening of > 5 mm was seen in 14 patients, and among them, 11 patients had pleural malignancy (p = 0.0038). In contrast, a study by Qureshi et al. [4] found pleural thickening > 1 cm in 14/33 patients, which was proven malignant on histocytology. They concluded that pleural thickening of > 1 cm was associated with malignant pleural effusion (p = 0.004). There was also a significant association between diaphragmatic pleural nodules and pleural malignancy, with 100% specificity and 60% sensitivity. Our study was comparable to Qureshi et al. [4], which demonstrated 100% specificity and 30% sensitivity of diaphragmatic nodules for pleural malignancy.
Conclusions
Transthoracic ultrasonography plays a vital role in the quantification of pleural effusion, assessment of the pleura and pleural space for pleural adhesions, thickening, and guiding trocar insertion. The Goecke formula for quantifying pleural effusion yielded an excellent regression coefficient. Pleural effusion echogenicity plays no role in predicting malignancy. Transthoracic USG is 100% sensitive and specific for detecting pleural septations in the presence of pleural effusion with 100% accuracy. The gliding sign is highly sensitive and specific for detecting pleural septations in the absence of pleural effusion. Pleural thickening is more sensitive, and the presence of diaphragmatic nodules is more specific for predicting pleural malignancies. However, our study had a small sample size of 31 cases. A study with a larger sample size can effectively establish its role in detecting malignancies. Moreover, a rigid thoracoscope, rather than a flexible thoracoscope, was used in our institution.