Introduction
Rotator cuff calcific tendinitis (RCCT) is a very common pathology, which presents in up to 7.5% of asymptomatic adults and up to 17% of patients with shoulder pain, caused by the deposition of calcium in the rotator cuff (RC) tendons [1]. This clinical condition occurs frequently in women, in about 60-70% of cases, especially between their 4th and 5th decades of life, and in 20% of cases the deposits are bilateral [2]. In 15-20% of patients, small deposits are usually asymptomatic, but symptoms, when present, may vary from sub-acute pain that worsens during the night to a severe and disabling condition associated with restriction of range of movements (ROM) resistant to anti-inflammatory drugs [3].
All tendons of the body may be affected by CT, but RC is the most involved site, and in particular [4]:
the supraspinatus, about 1 cm from its tendinous insertion on the grater humeral tuberosity, the so-called “critical zone area” (80%);
the lower side of the infraspinatus (15%);
the pre-insertional portion of sub-scapularis tendon (5%).
This clinical condition tends to resolve, often spontaneously. However, no consensus exists regarding treatment options. Patients with low-grade symptoms may be treated conservatively with rest, physical therapy, and oral non-steroid anti-inflammatory (NSAIDs) drugs while in patients with severe symptoms surgery can be replaced by new, less invasive techniques such as extracorporeal shock wave therapy (ESWT) and ultrasound-guided percutaneous irrigation of calcific tendinopathy (US-PICT) [5].
Aetiology and pathogenesis
The aetiology of CT remains largely unclear with multiple theories proposed. The process is probably related to a metaplastic transformation of tenocytes into chondrocytes that induce the deposition of calcific crystals inside the tendons [6]. Some authors correlate CT with a reduction of intratendinous oxygen afflux that may promote fibrosis and necrosis with subsequent fibre degeneration followed by calcific deposition [7]. Bishop and Bosworth individually proposed a theory based on the concept that repetitive tendons microtrauma leads to fibre degeneration followed by calcification [2,8]. Rui et al. postulated a relatively new theory based on an erroneous differentiation of tendon-derived stem cells (TDSCs) into bone cells, which leads to chondral metaplasia [9]. Recent studies focus their attention on the potential role of chondro-osteogenic BMP-2, BMP-4, and BMP-7 in the metaplasia of tendon cells leading to calcification [10].
According to Uhthoff et al., RCCT can be divided into 4 stages [11]:
first stage: precalcific stage, in which tendon metaplastic transformation in fibrocartilaginous tissue acts as a substrate for calcific deposits;
second stage: calcific formative stage, in which there is formation of single or multiple crystal deposition inside the tendons;
third stage: calcific resorptive stage, in which an increase in vascularity and oedematous infarction is seen with subsequent macrophages phagocytosis of deposits; the resorptive stage is usually associated with acute symptoms due to the oedema, which causes increased intratendinous pressure and, in some cases, the extravasation of crystals in the sub acromion subdeltoid bursa (SASD);
fourth stage: post-calcific stage: in which self-healing tendon repair by fibroblast requires several months. In that phase patients may present severe pain with reduction of ROM.
Imaging findings
Conventional radiography
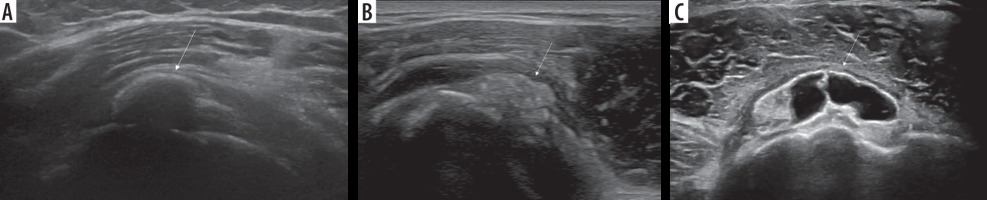
Conventional radiography (CR) is usually the first imaging modality for the investigation of shoulder pain. When RCCT is suspected, CR may detect the presence of calcifications in the projective site of the rotator cuff or in the subacromial space that confirms the diagnosis (Figure 1).
Figure 1
Conventional radiographic evaluation of left shoulder showing cloudy and dense calcification (arrow) of the supraspinatus/infraspinatus
projective sitesThe standard shoulder CR exam includes true anteroposterior view in internal, external, and neutral rotation of the arm and outlet view; although, an additional axillary lateral view can be helpful to detect calcification of subscapularis tendon [12].
Several classifications have been proposed based on the morphology, size, and radiographic aspect (Table 1), but all of them lack sufficient reproducibility [13].
Table 1
Classification of calcific deposits
Ultrasound
Ultrasound (US) is an established modality for the investigation of soft tissue pathologies [14]. On US, calcific deposits usually appear hyperechoic with or without acoustic posterior shadowing, due to the variable amount of calcific content [15]. Several classifications have been proposed in the literature but the 2 most important are Bianchi-Martinoli and Sconfienza:
Bianchi and Martinoli [16] described different calcification morphologies, based on the percentage of calcium:
type I calcification: hyperechoic foci with a well-defined acoustic shadowing;
type II calcification: hyperechoic foci with a mild acoustic shadow due to the lower amount of calcium deposit;
type III calcification: difficult to diagnose for their slightly isoechogenic pattern without posterior acoustic shadow.
Sconfienza et al. [6] classification is based on the degree of hardness usually encountered during a dry needling interventional procedure (Figure 2):
hard calcifications appear hyperechoic with strong acoustic shadowing;
soft calcifications appear hyperechoic or slightly isoechoic without acoustic shadowing;
fluid calcifications appear as hypo/anechoic deposits without acoustic shadowing.
Colour Doppler can be useful in the identification of the phlogistic phase [17] that occurs during the formative and resorptive stage with a direct correlation between Doppler signal and symptoms [18].
There has been innovation in progress regarding the utility of shear-wave sonoelastography, which could be useful for the evaluation of which calcific deposits might be treated on the basis of different hardness values.
Magnetic resonance
Magnetic resonance (MR) is considered the gold standard imaging modality for the evaluation of the shoulder, particularly in case of RC pathologies [19,20]; although the low number of resonating atoms of calcific crystal usually leads to low accuracy in the detection of CT [21].
On MR, calcifications generally appear as areas of low signal on all sequences within the tendon (Figure 3) [22].
Figure 3
Axial DP fat-sat image showing nuclei of low signal intensity (arrow) within subscapularis tendon. A phlogistic reaction of sub-acromialsub-deltoid bursa is also present
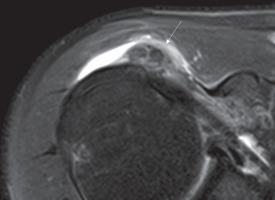
Nörenberg et al. [23] evaluated the diagnostic performance of susceptibility-weighted imaging (SWI) for the detection of CT in comparison to CR. These authors demonstrated 98% and 96% of sensitivity and specificity, respectively, in the identification of calcifications when compared with CR.
However, MR plays an important role in the evaluation of surrounding oedema, which is directly proportional to the patient’s symptoms, and in the detection of associated tendon tears. High signal intensity of oedematous changing could lead to a false positive findings of tears [6].
Conservative treatments
It includes manual physical therapy with exercises improving articular ROMs, and the administration of systematic NSAIDs in acute phase for the management of symptoms [24].
Injections of corticosteroids into the SASD bursa may be used to reduce the patient’s pain due to sub-acromial impingement and bursal inflammation.
If the pain lasts for more than 6 months, conservative treatment is considered to have failed.
Extracorporeal shock wave therapy
Extracorporeal shock wave therapy (ESWT) is a minimally invasive option for the treatment of calcific tendinitis [25]. The underlying mechanism of ESWT on RCCT is still debated, but the therapeutic mechanisms of ESWT seem to be caused by:
mechanical effect due to increasing pressure within the therapeutic focus that causes calcific fragmentation;
inflammatory effect due to mechanical irritation determining activation of proinflammatory factors with leukocyte recruitment and phagocytosis [26];
analgetic effect due to the inhibition of pain receptors [27].
Many studies have demonstrated that the therapeutic outcome of ESWT depends on the amount of energy applied, the number of treatments, and the different characteristics of the calcific deposit [28].
There is evidence that ESWT, in association with needling procedure, leads to higher therapeutic outcome compared with ESWT alone [29].
Ultrasound-guided percutaneous irrigation
In recent years, US-PICT has become the gold standard approach in cases of RCCT because of its safety, low invasiveness, and efficacy in calcific fading when compared to shock waves, because crystal deposits are drained outside the tendon [30-32]. This interventional technique brings significant pain relief with low incidence of minor complications such as vasovagal reaction and rare septic bursitis [33]. The procedure does not require any hospitalization and is performed under local anaesthesia, and for these reasons the patient can return home about 30 minutes after the procedure; furthermore, there is no need for immobilization, and patients return to their normal life 24 hours after the treatment. Surgical procedures may be associated with a significant psychological burden that potentially correlates with anxiety.
A recent study regarding patient experience during US-PICT reports mild procedural discomfort and overall satisfaction both immediately after treatment and after 3 months [34].
Well-demarcated hyperechoic foci with a weak posterior shadow or calcification with a central semiliquid consistency are the preferable deposit types dissolvable with US-PICT [35].
Not indicated calcification patterns include the following:
small calcification less than 5 mm;
fragmented calcification;
cluster of calcifications inside SASD;
intra-osseous migration of calcification.
Different approaches have been reported in the literature, which differ from each other due to the number of needles used.
The single-needle technique seems to be more appropriate for the treatment of soft or fluid calcifications [36] while the 2-needle approach might be more appropriate in cases of harder deposits [37]. Regarding the procedure, the patient is positioned semi-supine to prevent vasovagal reaction with the arm extended along the body in a slightly internal or external rotation according to the affected tendon. Subsequently, an antiseptic solution is used to prepare the skin and US probe, and up to 10 ml of local anaesthetic (usually lidocaine) is injected in the SASD bursa, near the calcific deposits and along the needle trajectory. All techniques required 16-gauge (G) needles, while smaller spinal needles of 18-20 G could be used to resolve obstruction inside the larger needle.
In case of the 2-needle technique (Figure 4), the first needle is inserted with an in-plane approach and parallel to the probe into the caudal portion of the calcification with the needle bevel open upwards, while the second needle is inserted into the upper portion of the calcification parallel and superficial to the first one, with its bevel in the opposite direction in order to create a close circuit [38]. Continual intermittent pressure is made to progressively fill the calcification with saline solution to dissolve its core.
Figure 4
Image of 2-needle ultrasound-guided percutaneous irrigation of calcific tendinopathy (US-PICT) procedure showing needle tips (arrow) into the calcific deposit (asterisk)
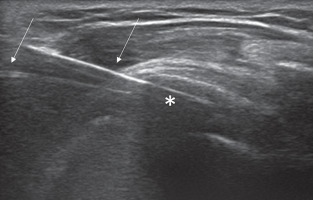
If a single-needle technique is used, the needle tip is inserted with an in-plane approach into the central portion of calcification, and an intermittent pushing procedure is done to wash out calcium material that refluxes inside the syringe [39].
In case of hard calcifications, the use of warm saline solution can reduce treatment duration, facilitate calcium dissolution, and reduce the incidence of post-procedural bursitis [40].
When only the calcification shell remains, a series of tendon perforations are performed under US guidance on the degenerated tendon to fragment the deposit and induce local bleeding, which promote growth factors.
At the end of the procedure an injection of low-solubility steroid into the SASD bursa is performed. The use of colour Doppler may help radiologists with less experience in injecting the drugs inside the correct site [41].
Conclusions
RCCT is one of the major causes of RC pain and disability, and it can be easily diagnosed with CR and/or US modalities. Even if resolution of calcific deposits occurs spontaneously, a safe and effective procedure for treating RCCT accelerating the reabsorption process. Radiologists must know this mini-invasive US-guided procedure as an alternative to surgical techniques, which are more invasive and prone to postoperative complications.