Introduction and methodology
Despite new diagnostic and treatment modalities in neurosurgery, aneurysmal subarachnoid haemorrhage (aSAH) still produces unfavourable outcomes. Severe haemorrhage, rebleeding, vasospasm, and cerebral ischaemia are factors that cause high mortality and morbidity [1-3]. Because death rates of 40-50% are common among patients with aSAH, prevention is considered a key approach [1].
Intracranial aneurysms (IAs), a source of aSAH, were previously reported to be found in about 3% of the general population [4,5]. Novel studies like the Tromsø Study [6] show us that the prevalence of IAs may be much higher. The study, utilizing 3-Tesla magnetic resonance angiography (MRA), found that 6.6% of the studied population (adults 40–84 years old) had intradural saccular aneurysms ≥ 2 mm. This number reaches 8.3% if including smaller (≥ 1 mm) and extradural aneurysms. Since 2005, the IA detection rate has increased from 1.7% to 3.4% [7]. It has been related to increased use of angiographic protocols for imaging modalities. Another study has shown that the percentage of positive CT angiography (CTA) for IA in a single centre in China was 4.8% [4]. The differences between studies are often attributed to different imaging modalities used for IA detection between studies.
High prevalence among the population and aSAH risk creates a necessity to consider preventive treatment of IAs. A decision regarding invasive treatment is made based on the thin-lined balance between IA estimated rupture risk and procedural risks. However, mortality and morbidity rates among treated patients – both endovascularly and surgically – can be up to 5% [3]. We need clear indications of when an IA should be treated invasively and when conservatively. The uncertainty is even higher in the case of small IAs (SIAs). Retrospective studies have found a significantly higher frequency of small, ruptured IAs than anticipated. On the other hand, natural history studies suggest a very low risk of rupture for SIAs.
Our goal was to summarise and compare all available data regarding diagnostics, treatment possibilities, and risks among IAs. To provide novel and up-to-date research we analysed both older, “canon” studies in the IA field and newer studies published in recent years. To choose recent impactful research for our review we identified important topics using the Peers for Peers platform created by the European Association of Neurosurgical Societies. The Consensus.app website was used to screen for impactful research in identified fields. Novel research regarding chosen areas was investigated via a PubMed search. Articles available via Peers for Peers were chosen based on the internal platform rating – only works classified as: “May change practice”, “Very influential”, and “Of general interest” in the category of “Aneurysm” were analysed. For this study we adopted a definition of SIA as IA with ≤ 7 mm dome size.
Small aneurysm, big problem?
Small IAs are defined based on their dome size regarding rupture risk related to its size. Three cut-off values are the most common in the literature: < 5 mm, < 7 mm, and < 10 mm [8]. Due to the dominance of SIA in overall IA presence it is wise to focus on the rupture rates of those because it has not been unified between studies.
The population-wide Tromsø Study [6] showed that 79.4% of unruptured IAs identified in their study were < 5 mm, while only 6.9% of IAs were ≥ 7 mm. The mean IA size among the studied population was only 3.81 mm. Other studies also show that the majority of all IAs are rather small (< 7 mm) [4]. This shows us the necessity to pay more attention to SIAs. However, the high prevalence of IAs sized < 5 mm may suggest lower rupture risk than previously estimated [6].
In 1998 the International Study of Unruptured Intra-cranial Aneurysms (ISUIA) [9] investigated IAs with dome sizes smaller than 10 mm and associated them with the occurrence of aSAH of 0.05% per year. The second publication of ISUIA assessed IAs < 7 mm with findings of annual rupture rates of 0% in the case of the anterior half of Willis circle IAs in patients without a history of previous aSAH. In those with a history of aSAH, the rupture risk was 1.5% per year [10]. New studies suggest that rupture rates of around 0% are real, but for SIAs with dome sizes of less than 1 mm in diameter [11]. The Unruptured Cerebral Aneurysms Study (UCAS) [12], another Japanese natural history study, followed 6697 aneurysms for 11,660 aneurysm-years. Both ISUIA and UCAS have defined cut-off points for low risk of rupture as 7 mm.
Furthermore, the Small Unruptured Aneurysm Verification Study (SUAVe) of the Japanese population focused on IAs smaller than 5 mm [2]. It showed a much higher than previously reported rupture risk of 0.54% per year. Despite being one of the key studies in the SIA field, we must consider the fact that the Japanese are known to have higher IA rupture rates [13]. During the SUAVe study [2] follow-up 30 of 448 (6.7%) aneurysms enlarged and 10 of 374 (2.7%) patients were operated on due to morphological changes. It is well known that after diagnosis, IAs can remain stable, grow, or rupture. The growth of an aneurysm is associated with an elevated risk of rupture that can reach 2-10% per year [14].
Another prospective cohort study focused on small IAs was published in 2013 [15]. Güresir et al. focused on IAs with dome size below 7 mm located in the anterior circulation. During the mean follow-up of 48.5 months, they found an annual rupture rate of 0.2%. Arterial hyper-tension and patient’s age below 50 years increased the rupture risk.
Research regarding SIA growth and rupture rates published until 2017 was summarized by Malhotra et al. [16]. The researchers found only poor-quality evidence suggesting that SIAs have low growth and rupture risk. Very small IAs (dome size below 3 mm) were reported to have almost no risk of rupture. SIAs of domes 5 mm and smaller had an annualized rupture rate of less than 0.5%, while those below 7 mm had a risk of less than 1%. On the other hand, Rutledge et al. [17] also showed that 75% of aSAH cases in their department were caused by SIAs (≤ 7 mm). The median ruptured IA size was 5.3 mm, and 48% of IAs were smaller than 5 mm. The median PHASES score was 5, corresponding to a 5-year rupture risk of 1.3%. An Australian retrospective study [18] investigating the 5-year experience of patients with ruptured IAs admitted to their institution showed that the mean maximum IA diameter was rather small – 6.4 mm. 90% of ruptured IAs had a diameter ≤ 10 mm and 73% ≤ 7 mm [18].
A study published in “Neurosurgery” in 2010 [19] shed some light on prospective follow-up on SIAs in terms of growth and rupture rate. The researchers investigated all studies on unruptured IAs published from 1966 to 2009 and selected all including IAs ≤ 7 mm for which measurements were done for at least 2 time points. Surpri-singly, such a vast analysis resulted in only 64 IAs that suited the criteria. During follow-up, 30 aneurysms ruptured, 27 of which were enlarged before rupture (90%). Thirty-four IAs remained unruptured, and 24 of them (71%) grew during follow-up. IAs that ruptured were found to be larger than unruptured ones [19].
To summarize, we can see that the reported rupture risk of IAs differed significantly among studies over the years with a large underestimation that has been given towards SIAs. Currently, the discrepancy between prospective and population studies remains tangled with prospective ones showing overall IA rupture rates of around 1.5% per year while population studies estimate them below 0.5% per year. What is more, rupture rates of SIAs remain questionable, especially in the 3-5 mm dome-size group, making preventive repair decisions even harder.
Diagnostics and assessment
Most IAs are discovered in asymptomatic patients while performing head imaging for other reasons. Incidental discoveries account for 91% of unruptured IAs diagnosed [12]. Detection and follow-up imaging suggested in 2015 guidelines [20] are CTA (computed tomography angiography) and MRA. Digital subtraction angiography (DSA) is considered useful only if invasive surgical or endovascular treatment is considered.
Different screening protocols have been suggested because novel studies show that people ≥ 35 years old with hypertension and smoking can have a lifetime risk of aSAH of up to 7% [21].
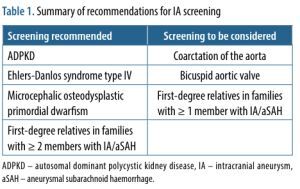
Screening for unruptured IA has been suggested in families with >1 affected person with an IA and in patients with several diseases, which increase IA development risk [22]. Screening recommendations are summarised in Table 1.
Table 1
Summary of recommendations for IA screening
Usually, CTA or MRA can detect IAs larger than 4 mm with high sensitivity [23-25]. Both imaging modalities also have a sensitivity of more than 90% for IAs larger than 2 mm in diameter [26]. Follow-up in patients treated conservatively should be considered with a form of regularly repeated MRA or CTA. The optimal time frame is not known, but a review by Brown et al. [22] suggested re-doingthe MRA or CTA on an annual basis for about 3 years, and then at a reduced frequency. For small unruptured and asymptomatic IAs of 2 to 3 mm in diameter, less frequent imaging can be suggested if an aneurysm is stable in consecutive studies. Recommendations are not specific about the time frames for follow-up imaging in treated IAs. MRA in general is less cost-effective and less available than CTA, and considering the similar sensitivity and specificity, it is reasonable to use CTA as the primary method to diagnose and follow detected and treated IAs [26].
It has been questioned that if repeated follow-up imaging does not improve the health of patients, should it be continued? That is the case for low-risk unruptured IAs [27]. However, determining ones with low rupture risk may be a challenging task.
Factors influencing rupture risk
In a worldwide meta-analysis of aSAH, the mean age of its occurrence was 52 years. It has also shown that the greatest influence, on the incidence of aSAH at the population level, is determined by mean systolic blood pressure and the prevalence of smoking. The incidence of aSAH decreases in the population with each decrease of 1 mm Hg in systolic blood pressure by 7.1%, and each percentage decrease in the prevalence of smoking corresponds to an aSAH risk decrease of 2.4% [28].
As mentioned above, the majority of ruptured IAs would be classified as SIAs. Some researchers have hypo-thesised that after rupture, the aneurysms shrink and become smaller [29,30]. This idea has been challenged – a case series has shown that most IAs increase their size after rupture [31]. Furthermore, another study showed that the domes of intact IAs were larger than those of ruptured ones [32].
The SUAVe study [2] explored factors that influence the risk of rupture of IAs smaller than 5 mm in dome size. It revealed that IA dome size ≥ 4 mm, patient’s age below 50 years, hypertension, and multiple aneurysms are factors that could suggest invasive treatment of microaneurysms. However, the study was carried out in the Japanese population, which could have influenced the results [2]. On the other hand, SIAs in patients with dyslipidaemia, hypothyroidism, and peripheral arterial disease were suggested to be more resistant to rupture [33]. Güresir et al. [15] showed that age below 50 years and hypertension were independent predictors of IA rupture. Another study suggested that in the event of enlargement of IA during follow-up, treatment should be proposed to the patient [20]. A study by Juvela et al. [34] that included a median follow-up of 21 years in patients with previous aSAH from other IA resulted in a further annual rupture rate of 1.1% per year. In the extended follow-up, smoking, IA presence on ACoA, and an IA diameter of 7 mm or less were predictors of haemorrhage. Furthermore, age was inversely associated with the risk of bleeding. Those studies suggest that in younger patients with comorbidities like hypertension, intervention should be more favoured than observation.
Burkhardt et al. [35] also summarised factors that influence the decision-making process in unruptured SIAs. They enumerated age below 50 years, Marfan syndrome, polycystic kidney disease, 2 first-degree relatives with IAs, previous SAH, and 5-7 mm dome size as features suggestive of preventative SIA repair. The authors suggested that clinical follow-up should be preferred in those > 70 years old, Japanese, Finnish, with second- or third-degree relatives with IAs, < 5 mm dome of IA, and localisation on the internal carotid artery (ICA) (in the cavernous segment). The family history of IAs may be a criterion for which it may be reasonable to treat even SIAs [20].
The anatomy and localisation of an IA can affect its risk of rupture. Morphology is an independent predictor of rupture, with a 3-fold risk increase in the case of irregular margins, 5.5-fold in IAs with a daughter sac, and 7.3 in multilobulated IAs (vs. single sac with a regular margin) [36]. The growth of an unruptured IA is another suggestive factor that the patient should undergo preventive repair [37].
The most common location of IAs in the Tromso Study was ICA. 42.7% of IAs were found on this artery. The anterior communicating artery (ACoA) – often discussed as the most prone to rupture – was the third most frequent localisation, at 10.7%. Overall, most IAs (85%) develop in the anterior part of the Willis circle, while 20% of patients have more than one IA [38,39]. Rupture risk is significantly higher with IAs of the ACoA and the ICA-posterior communicating artery (PCoA) complex, than with those of the middle cerebral artery (MCA) and ICA [12]. The frequency of IA development in each of the arteries has been extensively evaluated. ICA, MCA, and ACoA appear to be the most common parent vessels for the development of IA, but the results vary depending on the population included [32,40]. A review of ruptured IA locations has shown that ACoA IAs most often ruptured at sizes less than 10 mm (94.4%). Furthermore, many PCoA aneurysms frequently ruptured at sizes less than 10 mm (87.5%) [41]. Burkhardt et al. [35] found that localisation of an SIA in the posterior circulation, on MCA or ACoA, as well as IA bless/multi-lobulated form, are factors that favour preventive IA treatment.
One of the canon IA studies – ISUIA [9] – has been reanalysed in terms of morphological characteristics [42]. Perpendicular height and size ratio (maximum diameter to parent vessel diameter) were IA rupture predictors. Morphological parameters of SIAs < 5 mm were also assessed [43]. Aneurysm to vessel size ratio was associated with SIA rupture. ROC analysis resulted in a threshold size ratio separating ruptured and unruptured groups of 3.12. Anatomic features that occur more frequently in ruptured IAs are multi-lobulation, irregular dome margin, and the presence of a daughter sac [36]. The size of the parent vessel has also been evaluated as a possible predictor of the risk of rupture. The risk of rupture is greater for parent vessels with a diameter below 12 mm [32]. The dome-neck aspect ratio (AR) can also influence the risk of IA rupture. The odds of rupture can even be 20 times greater when the AR is > 3.47 compared to those with an AR of less than 1.38 [44].
Biomarkers of aneurysm development and rupture
The pathogenesis of IAs is complex and multifactorial, involving various genetic, molecular, and cellular mechanisms. A large meta-analysis [45] identified 19 single nucleotide polymorphisms associated with the presence of IAs: the cyclin-dependent kinase inhibitor 2B antisense inhibitor gene on chromosome 9, the SOX17 transcription regulator gene on chromosome 8, and the endothelin receptor A gene on chromosome 4 were the sites with the strongest connections. Circulating RNA in the blood has also been found to be useful in predicting the future growth rate of IAs, with genes related to injury, cancer, cardiovascular diseases, and cell-to-cell signalling being differentially expressed in high-risk IAs [46].
Additionally, vascular smooth muscle cells (VSMC) and lymphocytes are key cellular components in IA development and rupture risk [47,48]. When exposed to external stimulation, VSMCs can change their phenotype to an inflammatory one, resulting in matrix remodelling, which is a significant contributor to IA formation. Excessive wall shear stress (WSS) causes blood flow conditions that activate pro-inflammatory signalling in endothelial cells, which then recruits macrophages to high-WSS areas [49]. The inflammatory process is further perpetuated by lymphocytic infiltrates, which have been found in patient IA tissue. Inhibiting this inflammation process may be a crucial pharmacological therapy to halt the progression of IA. Moreover, the presence of interleukins was found to be associated with IA development risk and instability (Monsour et al. 2022, Liu et al. 2023), with TNF-α being one of the key factors in aneurysm formation and growth [50-52]. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were found to reduce the risk of IA and IA rupture [53].
Visfatin and nesfatin are novel biomarkers that may be useful for predicting the presence of aneurysm and subarachnoid haemorrhage, while the IL6/JAK/STAT signalling pathway and the oestrogen response pathway have been associated with the development of IAs [54,55] Another study has associated specific genes with aneurysm size of over 5 mm and high PHASES score; over 70 genes were found that reflected inflammatory signalling and vascular remodelling [56].
In summary, these studies provide insights into the complex mechanisms involved in the development, growth, and rupture of IAs. Identifying key genes, mole-cules, and cellular components can help improve risk stratification and treatment strategies for this life-threatening condition.
Scoring systems and treatment qualification
Precise patient and IA analysis requires extensive effort and sufficient time. As the 2017 report [57] showed, most physicians spend less than 16 minutes with each patient. It is not possible to extensively analyse all important factors and take into consideration the patient’s perspective, explain possible treatment solutions, and answer questions. Hence, scoring/rupture-risk estimating systems were found. The most popular model of prediction of IA rupture risk used among neurosurgeons is the PHASES score [3]. It allows us to estimate a 5-year rupture rate based on the nationality of a patient, hypertension, age, size of an IA, earlier aSAH, and site of the IA. Its use has been widely discussed and analysed, with the conclusion that a PHASES score of 3 is associated with a low probability of rupture of the IA, despite the low specificity of the classifier [58]. Moreover, a previously mentioned study of aSAH IAs by Rutledge et al. [17] showed that many low-PHASES-score IAs do rupture.
A study by Juvela [59] assessed the Unruptured Intracranial Aneurysm Treatment Score (UIATS) system [60] formulated by a multidisciplinary consensus team, and proposed a refined one. The UIATS system includes many factors, some of which do not properly correlate with the risk of rupture. The score increased with aneurysm size, but the study by Juvela showed that aneurysm domes < 4 mm had quite large rupture rates (29% in 20-year follow-up), almost as much as large ones. The risk of rupture increased to 38% as the aneurysms increased above 7 mm (up to 12.9 mm). The simplified scoring system proposed by Juvela S. included age, smoking, aneurysm diameter (≥ 7 mm), and aneurysm location [59]. Smedley et al. [61] also assessed differences between management suggested by a multidisciplinary team (MDT) and UIATS. The reliance of clinicians on IA size was an important factor of disagreement between assessors, again highlighting the importance of proper IA rupture risk analysis not solely based on IA dome size. Another novelty of this study is a comparison between the size of IA and the agreement of treatment between UIATS and MDT. Interestingly, appropriately treated IAs (with the agreement of both MDT and UIATS) treated conservatively had larger dome size than those appropriately treated through intervention. In another UIATS validation study [62], the UIATS recommended overtreatment of unruptured IAs as compared with the author’s preference. Although UIATS can be used as a screening tool, individualised treatment recommendations based on consultation with a cerebrovascular specialist are necessary.
A retrospective comparison between the UIATS and PHASES scores has suggested that the PHASES score has been much better at discriminating between ruptured IAs and unruptured IAs in their cohort. The lower discriminatory power of UIATS was due to the high weight of aneurysm-independent factors [63].
Another useful tool is the ELAPSS score [ 64], which has been found to predict the growth of IAs. External validation of this scoring system has proven its precision in estimating absolute 3- and 5-year risk for IA growth [65].
A large cohort of ruptured IAs was studied concerning their PHASES and ELAPSS scores [66]. A reasonable percentage of ruptured IAs would not qualify for intervention if decisions were based solely on these scores. The mean PHASES score for ruptured IAs was 5.3, and 17% of the ruptured ones had a PHASES score of 3 or less. The mean ELAPSS score for ruptured IAs was 13.89, and more than half of them had a low risk of future growth. Another study also evaluated the scores of the prediction tools in patients with ruptured IAs [67]. 46% of patients would have been assigned to the low- or very low-risk class by the PHASES risk calculator. About 28% of patients would have been assigned to a low-risk group, with a 3-year rupture risk below 1% according to UCAS investigators [12]. Although the ELAPSS score application showed a wider distribution between risk classes, 45.5% of the patients were in the low- or intermediate-risk class for aneurysm growth.
The studies allow us to have another insight into decision-making with unruptured IAs. Not all IA ruptures can be predicted, but we hope to get as close to finding a suitable rupture risk estimator as possible. As for now, those predictions require additional insight from an experienced neurosurgeon to pick a proper treatment modality for each patient and each IA. Those decisions are even harder in the case of small IAs, as additional factors like IA morphology and patient medical history must be included.
Dinger et al. developed a newly developed scoring system dedicated to small IAs [33], which includes both rupture risk and protective factors.
A comparison between UIATS, PHASES, simplified UIATS by Juvela S., and SIAAC scores is presented in Table 2.
Table 2
Scoring system comparison based on factors that suggest intervention or conservative treatment
[i] ↑ – factor suggests intervention/gives points that suggest intervention, ↓ – factor suggests conservative treatment/gives points that suggest conservative treatment, IA – intracranial aneurysm, aSAH – aneurysmal subarachnoid haemorrhage, ADPKD – autosomal dominant polycystic kidney disease, ACA – anterior cerebral artery, dACA – distal anterior cerebral artery, ACoA – anterior communicating artery, MCA – middle cerebral artery, ICA – internal carotid artery, PCoA – posterior communicating artery, BA – basilar artery
In the age of artificial intelligence and neural networks, there is an inflow of studies using those technologies to predict IA rupture. A recent study developed an impressive model that generated an area under a curve of 0.913 utilizing point cloud neural network prediction based on an IA model with parent artery involvement [68].
Moreover, an up-and-coming field is computational fluid dynamics (CFD) assessment. With more computational performance at our disposal than ever, we can do the following:
Despite rising computational power, CFD analyses are not available for each patient, due to insufficient performance of personal computers, and are reserved for cases requiring special attention and even more precise planning.
Treatment efficacy, safety and complications
Treatment of IAs and especially SIAs remains challenging in terms of both decision and skill. Today, no large randomised clinical trial (RCT) data define the optimal management of unruptured IAs. They can be managed conservatively or with an endovascular or open neurosurgical repair. Although some recommendations suggest microsurgical clipping as a first-line method for its durability with lower recurrence and retreatment rates, precise guidelines have not yet been established [35]. Qualification for each of the procedures requires an extensive analysis of patient- and IA-specific data and consideration of the benefits and risks of the procedure. What is more, endovascular treatment of SIAs has been reported to have an elevated complication rate in comparison to neurosurgical treatment of larger IAs [71].
The Nationwide Inpatient Sample database study [72] examined 7439 (53%) coiling and 6611 (47%) clipping procedures on SIAs. The death rates for patients who had coiling or clipping, respectively, were 2.17% and 2.66%, and morbidity rates equalled 2.16% and 4.75%. When modelled against the majority of natural history data, the adjusted risk of poor outcome shows a treatment advantage for clipping in patients under the age of 70 years and for coiling in patients below the age of 81 years.
There is still a lack of studies showing “real” complication rates for endovascular treatment including the risks of follow-up angiographies, recanalization of an IA, retreatment, and more. Surgical clipping requires less strict ambulatory control and, in most cases, does not require invasive follow-up imaging, apart from MRA or CTA. As for MCA aneurysms, the outcomes remain indifferent between endovascular and surgical groups, while clipping provided better occlusion rates [73]. ACoA IAs are yet another group often qualifiable for both approaches. A Finnish study [74] observed a recent transition from surgical to endovascular treatment. Although they did not find any major differences regarding complications and outcomes after the transition from a clipping-focused to an endovascular-focused approach, the raw numbers may allow us to draw some conclusions. Over time, the complication rate in surgical cases has decreased (from 29% to 17%) and it increased in the endovascular group (from 0% to 25%), with cerebral ischaemia being the most common in both groups. Overall, the permanent neurological deficit rate remained higher for the endovascular group (9% vs. 5%, respectively, for clipping) [74].
Endovascular treatment of SIAs has been explored. A metanalysis of ≤ 3 mm ruptured and unruptured IAs showed that the rate of mortality and morbidity is not negligible (7.3%). However, extrapolating the results of this study to unruptured SIA should not be done directly. 12.6% of all treated SIAs in that study had an incomplete or failed occlusion in an immediate post-coiling angiography, and 89% of those were unruptured SIAs. A follow-up angiogram showed that all of those progressed to complete occlusion. Among all SIAs, 91.4% were completely or nearly completely occluded [75].
Retreatment rates for IAs treated via endovascular coiling also support the thesis, with almost 10% requiring it due to recanalization. Two types were investigated: de novo bleb formation and enlargement of the residual cavity, with the former being smaller and having a longer time to retreatment. The authors concluded that in cases of even small IAs of anterior circulation after coiling the follow-up should be precise and constant [76]. This suggests the necessity to offer endovascular treatment to patients who are going to strictly participate in follow-up checkups for many years. Clipping as a more definitive treatment does not require such a precise observational routine.
Both clipping and endovascular procedures are exposed to the risk of intraprocedural aneurysm rupture (IAR). Coiling IAR rates vary between studies from 1 to 5%, while its prevalence is higher for clipping procedures. It is worth noting that in IAR cases during coiling, mortality rates are higher than during clipping [77].
A meta-analysis [78] based on pooled data has shown that patients with unruptured IA, who had undergone a clipping procedure experienced a higher rate of poor outcomes and bleeding compared to those with coiled IAs. On the other hand, Jiang et al. [78] showed that the incidence of complete occlusion was higher (OR 4.42) in clipped IAs. Clipping leads to a higher risk of intraoperative bleeding complications [78]. No special consideration was provided for small IAs in the above-mentioned studies. Long-term outcomes were found to be similar between procedures, with higher retreatment rates in coiling cases [79,80]. A preliminary report of an ongoing RCT (ClinicalTrials.gov Identifier: NCT01139892) that compares surgical clipping with endovascular coiling for unruptured IAs showed that the one-year risk of poor outcome (modified Rankin Scale > 2) after preventive occlusion ranged from 3.6% to 4.2% for both modalities, without statistically significant differences between them [81]. A high-volume study performed by Alshekhlee et al. [82] revealed higher in-hospital mortality in patients undergoing clipping in comparison to the endovascular group (adjusted OR equalled 3.6). Perioperative intracerebral haemorrhage and acute ischaemic stroke were found more often in the clipping group.
The most important period is the close follow-up and postop imaging done routinely within 24 hours. That may identify any complications that do not present clinically, like clip slippage. One study reported that this complication occurs in 2.6% of clipped IAs. They identified lower clip closing force, single clip, and oversized clip length as risk factors for clip slippage [83].
High-risk criteria associated with negative outcomes have recently been defined for IA clipping, as follows: age at surgery above 65 years, IA of the posterior circulation, IA diameter above 10 mm, IA calcification, irregular IA configuration, chronic obstructive pulmonary disease, coagulopathy or bleeding disorder, history of congestive heart failure, history of stroke, American Society of Anaesthesiology physical status IV or higher, and body mass index above 40 kg/m2 [84].
Implementation of novel devices in a neurointerventional practice has widened the range of possible solutions for small or complicated IAs. One of them – the Flow Redirection Endoluminal Device (FRED) – is a new tool that has gained popularity among neurointerventionalists in recent years. A North American multicentre study [85] has recently taken a deep look into FRED procedures. Despite a 99.2% successful deployment rate in the studied group, complete occlusion after at least 10 months of follow-up was achieved in only 48.8% of cases. The method was used for small, large, and giant IAs with a mean dome size of treated IA equal to 7.2 mm. The size of the IA did not correlate with the occlusion rate. Neck size and patient age were found to correlate negatively with occlusion rates. The complication rate was also found to be quite large – 26.7%. The morbidity rate reached 8.6%, and 3.6% of patients had an mRS score > 2 at the final follow-up (vs. 0.9% before treatment) [85]. This study reported higher complication rates than its European and South American counterparts. The authors concluded that FRED devices should be sensibly used regarding their findings, and further prospective studies should determine “real” FRED treatment outcomes and complications.
Woven EndoBridge (WEB) devices were introduced into practice almost a decade ago. One multicentre study [86] summarized the data gathered during that time. It found that smaller IAs were treated with WEB devices. The analysed data also showed a significant decrease in the neck sizes of those IAs. Over time, neurointerventionalists tended to treat patients presenting with ruptured aneurysms. Usage of WEB devices allowed for adequate occlusion in 85.7% of IAs, and through the years lower retreatment and complication rates. However, thromboembolic and haemorrhagic complications were encountered in 7.5% and 3.0% of procedures. Although most WEBs were placed in unruptured IAs, the study does not draw any conclusions regarding the safety and efficiency of such devices in unruptured SIAs.
A change of paradigm may be possible in IA treatment because a large study is about to begin with a protocol already published. The Risk of Aneurysm Rupture (ROAR) study [87] will be a longitudinal multicentre study with a target sample of 20,000 IA patients. However, due to the size of the study, it may require up to a decade or more to draw any conclusions.
Conclusions
The vast majority of retrospective, prospective, and so-called “natural history” studies suggest that most ruptured IAs are relatively small (≤ 7 mm). It shows the growing need for research focused on IAs sized between 3 and 5 mm. For larger IAs, the indications are clear and well established, with the greatest emphasis on IA size. In the case of small IAs, size differences may be of lesser significance. IA and parent vessel aspect ratios, morphology, location, family history of aSAH, comorbidities like hypertension, smoking, alcohol abuse, and patient’s age are some of the key aspects in terms of preventive IA repair decisions.