Introduction
Elbow pain and immobility may be debilitating. Aetiologies often include inflammatory arthritis (particularly rheumatoid arthritis [RA]), trauma, and primary as well as secondary osteoarthritis (OA). Additional causes such as osteonecrosis, infection, and crystalline arthropathy (calcium pyrophosphate and uric acid deposition) are less common but may be equally disabling.
Joint arthroplasty is reserved as definitive therapy for cases of severe pathology, disability, and failed conservative management. The use of total elbow arthroplasty (TEA) has almost doubled between 1998 and 2011 in the United States [1]. However, it is a relatively uncommon surgery with a rate of 1.4 per 100,000 people annually versus 70-99 per 100,000 annually for hip arthroplasty [2,3]. TEA hardware can have varying degrees of constraint (Figure 1). In addition to TEA, hemi-arthroplasty, interposition arthroplasty, and resection arthroplasty also play roles in the management of elbow pain (Figure 2). Medical imaging, to include radiographs, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), and occasionally nuclear medicine imaging, helps identify the aetiology of elbow dysfunction, document the severity of imaging findings, and guide perioperative management.
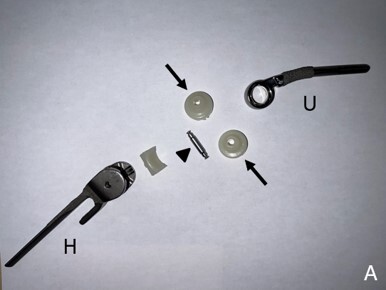
Figure 1
Example of a Zimmer-Biomet total elbow prosthesis. (A) Deconstructed and (B) assembled components comprising an ulnar stem (U), humeral stem (H), polyethylene hinge bearing (arrows), and linking pin (arrowhead). This prosthesis is considered a “linked” prosthesis because the humeral and ulnar components are associated via a hinge mechanism
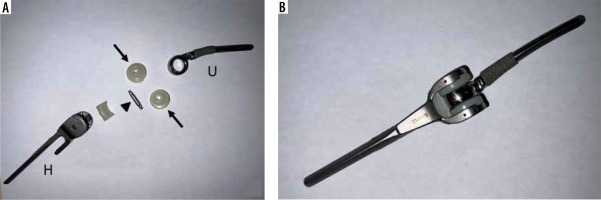
Figure 2
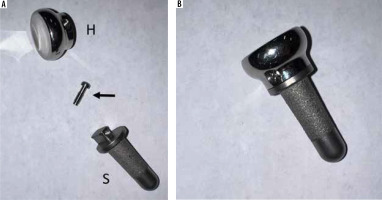
Example of a Zimmer-Biomet radial head arthroplasty. (A) Deconstructed and (B) assembled components comprising a radial head (H), locking bolt (arrow), and stem (S). The radial head is secured to the stem via a locking pin
This article reviews the different types of elbow arthroplasties, their indications, their normal postoperative imaging appearances, and the imaging findings of complications. A comprehensive understanding of elbow arthroplasty and its complications is necessary to provide timely and accurate diagnosis on imaging and establish optimal patient care.
Clinical indications for elbow arthroplasty
The elbow is the second largest diarthrodial joint in the upper extremity and consists of articulations between the radius, humerus, and ulna (Figure 3). The ulnohumeral articulation is a hinge joint between the olecranon of the ulna and trochlea of the humerus; it affords elbow flexion and extension. The radiocapitellar articulation is between the concave surface of the radial head and the more convex surface of the humeral capitellum. The radial head rotates on the capitellum to allow supination and pronation of the forearm. Normal ranges of motion are 100 degrees of flexion – 30 degree maximal extension to 130 degree maximal flexion – and 100 degrees of forearm rotation – 50 degree maximal pronation to 50 degree maximal supination.
Figure 3
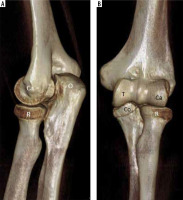
3D reformatted images of the elbow (A, B) demonstrate normal elbow anatomy. R – radial head, Ca – humeral capitellum, O – olecranon process of the ulna, Co – coronoid process of the ulna, T – humeral trochlea
The elbow is involved in approximately 50% of patients with RA, which is the most common indication for TEA [4,5]. Welsink et al. [6] reported RA as the indication for 70% of the TEA performed in a systematic review of 9379 patients. Joint capsular thickening, synovitis, and destructive osseous erosions are found in the rheumatoid elbow. Pain throughout the entire range of motion of the elbow is typical for this patient population, with limited range of motion and instability occurring in more advanced disease.
Joint space narrowing, osteophyte formation, subchondral sclerosis, and cyst-like changes are typical of OA. Elbow OA may be primary or secondary. Primary OA typically occurs in athletes or manual laborers secondary to repetitive overuse and stress. Pain usually occurs more commonly at the extremes of the range of motion and is more typical of advanced disease. Osteophyte formation can cause impingement symptoms. Secondary OA occurs in the setting of prior trauma, inflammation, or infection.
The goal of therapy for patients with elbow dysfunction is to restore mobility, decrease pain, and minimise morbidity. With the exception of severe trauma, conservative management is initially used, including physical therapy, rest and activity modification, splinting, anti-inflammatory medications, and anti-rheumatic/biology therapies when appropriate. Resolution of signs and symptoms of RA is achievable in 10% of patients receiving anti-rheumatic drugs and biologic therapies [7]. Surgical management is indicated for cases refractory to conservative management and/or with severe disease and functional limitations.
Types of elbow arthroplasty
Arthroscopic or open synovectomy, with or without radial head excision, is indicated for early disease stages of RA to decrease pain and prevent joint destruction. The advantages of arthroscopic synovectomy include a shorter length of rehabilitation, better visualisation of pathology, and decreased patient morbidity. However, recurrence of synovitis and progression to TEA are not uncommon [8-10].
Arthroscopic or open debridement – also known as ulnohumeral or osteocapsular arthroplasty – consists of osteophyte and intra-articular body removal, and it is indicated in patients under 60 years of age with elbow OA who have higher functional needs. The anterior capsule is sometimes released to improve the range of motion. Satisfactory pain relief and range of motion are achieved with low complication rates [11-13].
Interposition arthroplasty is indicated for severe disease in patients younger than 30 years of age with RA and in patients younger than 60 years of age with OA. This is a valuable option for younger, more active individuals because it does not require the weight-lifting restrictions of TEA. Achilles tendon allograft and fascia lata autograft have been used with success [14,15]. The graft is affixed to the distal humerus with extra material available for collateral ligament reconstruction, as needed. This is of particular importance in patients with RA with prolonged damage to the soft tissues of the joint from prolonged synovitis and usage of corticosteroids.
Trauma is the primary indication for radial head hemiarthroplasty. Radial head fractures account for 33% of all elbow fractures in adults and often present with ligamentous injury and elbow instability [16]. Radial head fracture was the indication for 98.4% of a cohort of 970 radial head hemiarthroplasties performed at one health care system [17]. Over 50% of this population also had concomitant ligamentous repair. First performed by Speed in 1941 using a ferrule cap, radial head arthroplasty is indicated in complex radial head fractures where the native radial head cannot be satisfactorily reconstructed, and in instances of ulnar collateral ligamentous injury [18]. Titanium or cobalt chrome is currently the material of choice, improving upon the stability and durability concerns of silicone. Implants can be unipolar or bipolar, which has a radial head component that articulates with the radial neck via a polyethylene-metal bearing. One primary disadvantage of radial head arthroplasty is the development of symptomatic OA of the capitellum (Figure 4). Capitellar hemiarthroplasty is a relatively newer technique, first described in 2008, which aims to reduce this complication [19]. Humeral hemiarthroplasty is uncommon and not utilised in our practice.
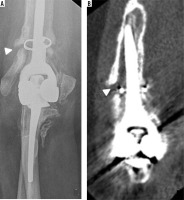
Figure 4
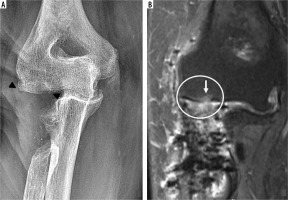
Frontal radiograph of the elbow (A) status post radial head hemiarthroplasty hardware removal secondary to pain demonstrates small marginal osteophyte formation on the capitellum (arrowhead), in keeping with capitellar osteoarthritis, which was confirmed intra-operatively. Coronal PD FS MR image of the same elbow (B) demonstrates high grade cartilage loss (circle) over the capitellum with subchondral reactive marrow change (arrow)
TEA is reserved for patients experiencing severe pain and functional deficit refractory to conservative or minimally invasive treatment. The consensus minimum age for TEA is 60 years with the exception of patients with RA, where the age limit is 30 years. Involvement of the ipsilateral upper extremity is seen in 80-90% of patients with RA of the elbow [20]. Therefore, it is assumed that these patients will place less stress across the elbow joint, prolonging the life of the prosthesis. Strict weight-lifting restrictions are imposed after TEA; 5-10 lbs total weight or repetitive lifting of 2 lbs. Inability to follow these guidelines is a contraindication for TEA.
Unsatisfactory rates of hardware loosening and joint instability occurred with the non-cemented elbow arthroplasty designs of the 1940s and 1950s [21]. The first cemented TEA was performed by Dee in 1972 [22]. Early rigid-hinged, constrained prostheses demonstrated a high rate of hardware loosening and periprosthetic fracture [21]. The soft tissue release that accompanied these replacement surgeries contributed to this complication, as all forces were subsequently transferred across the bone and hardware rather than being offset by a competent soft tissue envelope [23,24]. Over the years, an improved understanding in elbow biomechanics, prosthesis fixation and bone preparation techniques, and clinical outcomes of prior elbow arthroplasty led to hardware modifications that are still in use today.
Unconstrained TEA consists of separate humeral and ulnar components that articulate via a polyethylene bearing surface (Figure 5). This design requires a competent soft tissue envelope. Because of this requirement, forces across the elbow are transferred through the soft tissues, and these designs historically have the lowest rate of hardware failure. However, since there is no pin that links the components, this design is inherently more unstable than other models.
Figure 5
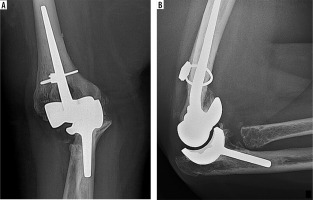
Frontal (A) and lateral (B) radiographs of the elbow show an unconstrained total elbow arthroplasty with a radiolucent polyethylene bearing surface interposed between humeral and ulnar components with no physical link between them
The semi-constrained or partially hinged design shares features of both the constrained and unconstrained designs, giving satisfactory stability without overloading the cement/bone interface. The “loose hinge” affords 7-10 degrees of varus/valgus movement, which is more in line with normal elbow kinematics than the rigid design. This is the most commonly used TEA design today, consisting of a titanium or cobalt chromium ulnar and humeral components that are linked with a pin and polyethylene bushing to limit friction (Figure 6). The hardware comes in mono-block or modular forms. The modular form is useful in cases when greater than normal bone loss is expected (e.g. tumour resection and reconstruction) (Figure 7). Most semi-constrained designs have an anterior flange on the humeral stem to reduce rotational stress on the bone-cement interface.
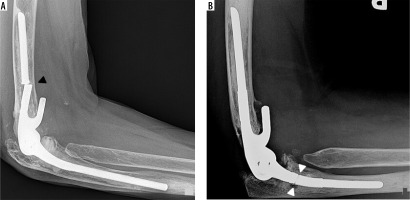
Figure 6
Lateral (A) radiograph of the elbow shows a semi-constrained TEA. Arrow – anterior flange of the humeral component. Image B is a magnified coned down frontal radiograph of the hinge aspect of the hardware, showing the linking pin (dashed line) and polyethylene bushings (under arrowheads)
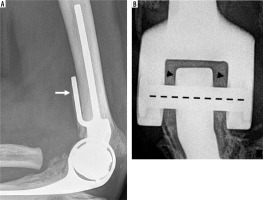
Figure 7
Frontal radiograph of the humerus (A) demonstrates a modular semi-constrained TEA connected to a megaprosthesis shoulder arthroplasty in a patient who underwent revision surgery for failed TEA secondary to non-healing periprosthetic fracture. Lateral radiograph of the elbow (B) demonstrates a modular semi-constrained TEA in another patient with limited humeral bone stock status post trauma
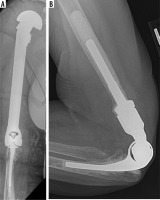
Ten-year survival rates of the semi-constrained design are over 90%. The Mayo Elbow Performance Score (MEPS), originally published by Morrey, combines the objective measurements of elbow range of motion and stability with the subjective assessment of pain and daily functioning [21]. Scores range from 0 to 100 and include excellent (90-100) and good (75-89) outcomes. Sanchez-Sotelo et al. [25] reported the long-term outcome of 461 semi-constrained TEA in patients with RA from 1982 to 2006. Eighty-nine per cent of patients did not need hardware revision or removal on final follow-up. The mean MEPS of these patients was 90, including 87% reporting no or mild elbow pain. A case series of 78 semi-constrained TEAs demonstrated a MEPS of 89 on 5-year follow-up for patients with inflammatory arthritis compared to 80 for post-traumatic indications [26].
Normal imaging findings
Initial post-operative assessment of all types of elbow arthroplasty begins with radiography. The hardware stems should be centred within their respective medullary canals. Lucencies less than 2 mm at the hardware bone or cement bone interface can be within normal limits but should be followed on subsequent imaging [16]. Cross-sectional imaging has additional advantages in hardware and soft tissue assessment. MRI is particularly useful in evaluating the marrow space around the hardware; T1 and proton density (PD)/T2 signal in the periprosthetic marrow should be similar to surrounding marrow. Joint effusion and soft tissue swelling on radiography are more completely assessed on cross-sectional imaging. These may be within normal postoperative limits; however, they merit close attention on follow-up imaging, as clinically indicated. In the setting of expected infection or particle disease/metallosis, sampling of these collections is warranted.
Complications and imaging findings of complications
The most commonly used imaging modalities to assess for complications of the elbow arthroplasties are radiography, CT, and MRI (Figures 4-23). Nuclear medicine imaging is also used. US may be particularly useful in the evaluation of triceps tendon and ulnar nerve postoperative complications with appropriate examiner expertise.
Irrespective of design, the complication rate of modern TEA has been reported to be 20-40% [27]. When separating by design type, the complication rate of the semi-constrained design has been reported to be 10% compared to 50% in the constrained and unconstrained versions [23]. The reported complication rate of radial head arthroplasty is 23% [28]. Welsink et al. [6] conducted a systematic review of 9379 TEAs and reported rates of aseptic loosening (7%), deep infection (3%), periprosthetic fracture (3%), ulnar nerve palsy (3%), and instability (2%) on follow-up between 3 and 12 years. Voloshin et al. [27] conducted a separate review of 1981 TEAs from 38 separate studies and reported complication rates of clinical loosening (4.8%), instability including dislocation and symptomatic subluxation (3.8%), deep infection (2.5%), and ulnar nerve complication (2.5%). Other types of complications following elbow arthroplasty include wound healing, ectopic bone formation, and particle disease. Polyethylene bushing wear and triceps insufficiency are also encountered.
Loosening
Aseptic loosening is the most common complication of elbow arthroplasty. With respect to TEA, the ulnar component is more commonly involved, secondary to an anterior distracting force from a variety of aetiologies: heterotopic bone, coronoid process, flange of the prothesis, and scar tissue [29-31]. The incidence of loosening correlates with the degree of device constraint with 5-year loosening rates of 25%, 6-17%, and less than 2% for constrained, semi-constrained, and unconstrained implants, respectively [20]. Loose humeral or ulnar stems can generally be replaced with longer stems in revision surgery. Strut allograft augmentation and cerclage wire fixation can add bone stock and structural integrity to areas of cortical deficiency. The incidence of loosening with radial head arthroplasty has been reported to be 29% [32].
Loosening on radiography or CT is denoted by a lucency of greater than 2 mm at either the bone-prosthesis, cement-bone, or cement-prothesis interface (Figure 8). It is important to note that hardware infection can present with these imaging findings and must be differentiated from loosening through clinical examination, laboratory workup, and patient history. Advanced cases of loosening can result in cortical deficiencies and sometimes extraosseous protrusion of hardware (Figure 9). Comparison with prior radiographs is beneficial in detecting subtle differences in prosthesis orientation in cases of early component subluxation and translation (Figure 10).
Figure 8
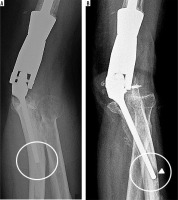
Two lateral radiographs (A, B) of the elbow demonstrate posttraumatic deformity of the elbow in a patient treated for advanced secondary osteoarthritis post total elbow arthroplasty. Note the progressive lucency at the hardware-bone interface of the ulnar stem on follow-up radiograph (B – arrowheads) measuring greater than 2 mm in width, in keeping with hardware loosening. Coned down frontal elbow radiograph (C) in another patient with lucency about the distal aspect of the radial head hemiarthroplasty stem (arrowhead) and corresponding periprosthetic increased signal on subsequent coronal STIR MR image (D – asterisk). Radial component loosening was confirmed intra-operatively
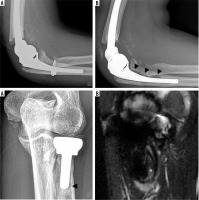
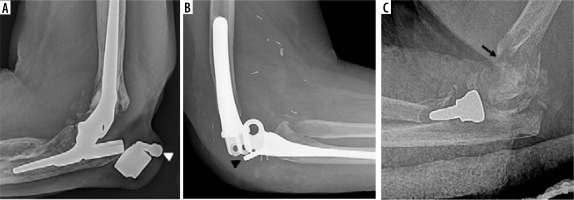
Figure 9
Lateral elbow radiograph (A) and sagittal reformatted CT image (B) of the elbow demonstrate pronounced periprosthetic osteolysis and loosening of the ulnar and humeral components (B, C – asterisk). Note cortical deficiency of the humerus with extra-osseous protrusion of the humeral stem (A, B – arrowhead)
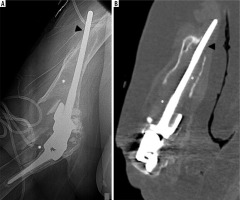
Figure 10
Two frontal radiographs of the elbow demonstrate interval increase in lateral migration of the ulnar stem on radiographs obtained one year apart (circle). Note the distance between the tip of the ulnar stem and lateral cortical margin has decreased in image with new periprosthetic lucency (arrowhead)
Periprosthetic joint infection
It is postulated that increased rates of periprosthetic joint infection (PJI) in elbow arthroplasty compared to other joint replacements are secondary to the relatively subcutaneous location of the joint and the concomitant use of immunomodulating drugs in patients with RA [20, 21, 33]. The most common pathogen is Staphylococcus epidermidis followed by methicillin-sensitive S. aureus and Klebsiella pneumoniae [34]. Staphylococcus epidermidis attaches to and colonises orthopaedic hardware via a biofilm, which shields the bacteria from host defence and antibiotic therapy. Infection with S. epidermidis portends a poorer prognosis. Serum inflammatory markers (e.g. C-reactive protein and erythrocyte sedimentation rate) have shown limited correlation with confirmed elbow PJI, and patients often have a normal white blood cell count [35]. Implant sonication to disrupt biofilm and synovial fluid and cytokine analysis (e.g. interleukin-6 and alpha-defensin) have shown promise in detection of PJI in other joints; however, intra-operative culture is traditionally considered the gold standard [36-38].
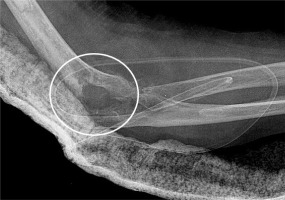
If PJI is detected within the first postoperative month and the components are deemed stable, the treatment of choice is hardware salvage with debridement and 6 weeks of intravenous (IV) antibiotic therapy. Two-stage revision surgery is indicated for subacute and chronic PJI, or with radiographic or intraoperative evidence of loose components. This consists of prosthesis explantation, insertion of antibiotic impregnated polymethylmethacrylate spacer, and re-implantation of hardware after 6 weeks of IV antibiotic therapy and negative cultures. Resection arthroplasty is considered in elderly patients with low functional demands. It is important to maintain the humeral condyles upon hardware removal because they are contoured to articulate with the ulna in resection arthroplasty to provide structural stability (Figure 11).
Figure 11
Lateral postoperative radiograph of the elbow demonstrates resection arthroplasty changes after removal of TEA hardware secondary to infection. The humeral condyle is contoured (circle) for structural stability as it articulates with the ulna
On radiography, progressive osteolysis at the bone-hardware interface or any lucent margin greater than 2 mm in thickness corroborates clinical suspicion of infection. Cross-sectional imaging can further evaluate for synovitis, joint capsule thickening, and the presence of abscess or phlegmon (Figure 12). US is most frequently utilised for image-guided aspiration. In addition, MRI is valuable in assessing the quality of the corticomedullary bone in instances of suspected septic arthritis and osteomyelitis. Three-phase technetium 99m-MDP bone scan and 18F-FDG PET/CT are commonly used nuclear medicine studies to evaluate infection and loosening. Both will show increased activity in the area of concern; this includes all 3 phases on bone scan.
Figure 12
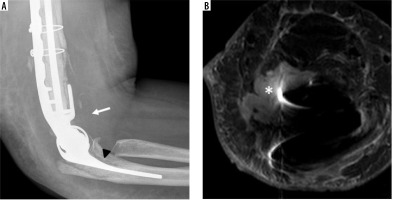
Lateral elbow radiograph (A) demonstrates peri-prosthetic lucency at the ulnar stem-bone interface (arrowhead) and elbow joint effusion (arrow) in this patient with suspected hardware infection. In addition to the joint effusion (not shown), axial STIR MR image (B) shows a fluid collection extending anterior and superior from the elbow joint (*), in keeping with soft tissue abscess confirmed intraoperatively
Periprosthetic fracture
Loosening and infection can contribute to periprosthetic fracture. The Mayo Clinic classification system of periprosthetic fracture is useful in helping guide treatment. Fractures that are outside the margin of the hardware (Type 3) usually do not require surgical revision [39]. Conversely, fractures that involve the humeral condyle or olecranon (Type 1) or the prosthesis stem (Type 2) usually require hardware revision surgery. It is preferable that the stem of the revised hardware and any allograft bypass the fracture site by at least 2 cortical widths [40].
Imaging findings of fracture include linear lucency on radiography or CT or a linear signal abnormality on MRI surrounded by bone marrow oedema (Figure 13). Fractures can be nondisplaced or with cortical step off at the fracture plane with varying degrees of angulation.
Instability
Improvements in operative technique and implant design have reduced the incidence of instability. The integrity of the collateral ligaments and remainder of the soft tissue envelope contribute to elbow stability following TEA. An incompetent soft tissue envelope can contribute to elbow dislocation. Immediate postoperative instability is rare. Asymptomatic instability is not uncommon and rarely needs treatment. The first line of treatment for symptomatic instability is immobilisation of the elbow in flexion for 3-6 weeks with consideration for revision to a semi-constrained device reserved for refractory cases [20].
Ulnar neuropathy
Ulnar neuritis following TEA ranges from profound neuropathy to transient paraesthesia. Individuals with RA and history of prior elbow surgery are particularly vulnerable [21]. Causes are suspected to be excessive traction, direct mechanical pressure, and epineural/perineural haematoma formation. Routine in situ decompression of the ulnar nerve is common practice to attempt to mitigate these issues. Some surgeons reserve transposition of the ulnar nerve for cases of potential nerve compromise from osseous deformity while others routinely perform transposition. Voloshin et al. [27] in their meta-analysis compared the rates of postoperative ulnar neuropathy between routine and non-routine ulnar nerve transposition and found no statistically significant difference; however, the lower rate of ulnar neuropathy in the cohort that practiced routine ulnar nerve transposition may have clinical implications.
Nerve conduction studies are valuable in the localisation of ulnar nerve lesions; however, cross sectional imaging can play a role in identifying regions of neural compression and mass affect (Figure 14). Change in calibre of the ulnar nerve or acute angulation on US and MRI have correlated with intra-operative findings of ulnar neuropathy in patients who have undergone transposition for additional indications [41].
Figure 14
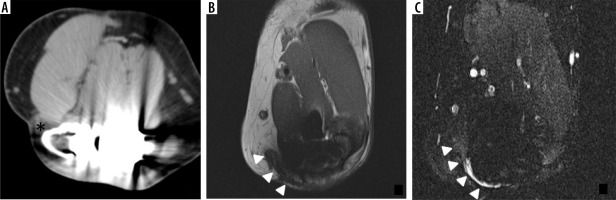
Axial CT image (A) in a patient who underwent TEA shows increased density about the subcutaneously transposed ulnar nerve (asterisk). Axial T1-weighted (B) and STIR (C) MR images demonstrate postsurgical distortion of the soft tissue plane and an angular orientation of the ulnar nerve (B, C – arrowhead) with STIR signal hyperintensity at the posterior aspect (C) in this patient presenting clinically with ulnar neuropathy status post TEA
Wound healing
Individuals in whom TEA was performed for advanced posttraumatic OA have often had prior instrumentation of the joint with disruption of local soft tissue integrity and tenuous blood supply, which can contribute to wound healing complications. Delayed wound healing and prolonged, spontaneous wound drainage (longer than 10 days after operation) are associated with increased rates of elbow PJI [42]. Wolfe et al. [42] conducted a risk-factor analysis of 12 cases of infected TEA from a cohort of 164 elbows at a single institution and demonstrated no association between PJI and the indication for surgery (RA vs. trauma). However, individuals with RA involvement of the ipsilateral shoulder did have higher rates of wound healing complication.
Particle disease
Prosthesis wear can incite particle disease, a local inflammatory process from shed prosthesis foreign particles. This can lead to implant loosening and failure (Figures 15 and 16) [43]. This includes component separation and dislocation (Figure 17). During revision elbow arthroplasty, Day et al. [44] studied tissue samples of the capsule and medullary bone in patients undergoing revision elbow arthroplasty for osteolysis and component loosening and found polyethylene, cement, and metal debris.
Figure 15
Two lateral radiographs of the elbow in a patient treated for a displaced and angulated fracture of the radial head/neck with subsequent radial head hemiarthroplasty (A). B) Periprosthetic lucency developed adjacent to the stem (arrow) and head (arrowhead) of the radial prosthesis on follow-up imaging 2 years later, in keeping with particle disease and loosening
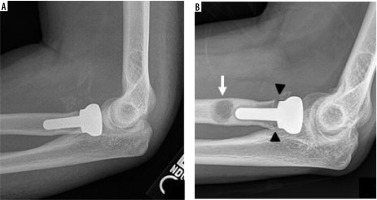
Figure 16
Frontal radiograph (A) of the elbow demonstrates deficiency of bone about the stem of the radial hemiarthroplasty hardware (arrowhead) and increased density along the lateral margin of the elbow (asterisk). Coronal T1-weighted (B) and axial PD-weighted (C) MR images further characterise the density as a peri-articular fluid collection (B, C – asterisk) with inflammatory changes (B, C – arrowhead) in this patient with histology proven particle disease
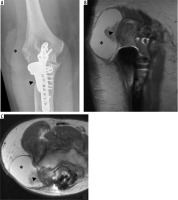
Figure 17
Lateral radiographs of the elbow in 3 different patients following TEA (A, B) and radial head replacement (C) with component separation and dislocation. Note the displaced locking pins (A, B – arrowhead). Comminuted distal humeral fracture is present in image C (arrow)
Osteolysis on radiography presents as amorphous lucent regions surrounding the hardware, often progressive on subsequent radiographs. Associated component loosening is not uncommon. CT and especially MRI can better characterise the degree of soft tissue inflammatory change.
Heterotopic bone formation
Heterotopic bone formation around the elbow is relatively rare, but it can inhibit patient motion, particularly in flexion. This can lead to component loosening, and it can be readily assessed with radiography (Figure 18).
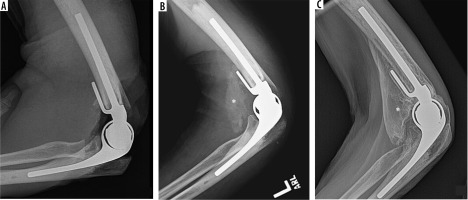
Figure 18
Lateral radiographs of the elbow status post TEA (A – immediate post op, B – 2 months post operative, C – 3 years post operative) demonstrate progressive maturation and size of heterotopic ossification (B, C – asterisk) at the anterior aspect of the elbow in this patient with reduced range of motion upon elbow flexion
Hardware fracture
Fracture of TEA hardware is rare because the periprosthetic bone usually fractures before the hardware fails. However, silicone implants used in radial head hemiarthroplasty have high rates of loosening and fracture [45,46]. Because of the density of silicone, MRI is particularly useful in identifying the hardware fracture and can also check for silicone-induced synovitis (Figure 19). Hardware fracture most commonly occurs in the stem of the prosthesis and necessitates surgical intervention (Figure 20).
Figure 19
Lateral radiograph of the elbow (A) demonstrates postsurgical changes related to radial head resection with hemiarthroplasty. Note fracture of the silicone radial head implant with posterior displacement (arrowheads). Axial (B) and sagittal (C) PD-weighted MR images of the elbow show the displaced radial head component (B, C – asterisks) in relation to the stem (C – arrow). Note the lack of MR artifact from the silicone implant and elbow joint effusion with synovitis (B – arrowhead)
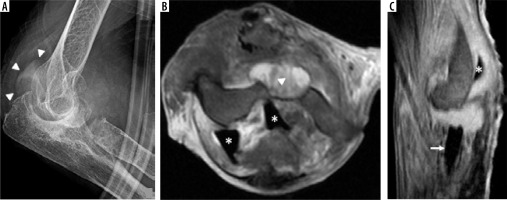
Complications of neoplasm
Similarly to MRI, PET CT demonstrates high sensitivity in disease localisation, which can be useful not only in the detection of infection but also in tumour detection (Figure 21). The non-attenuation corrected images are particularly useful in these cases because of the known artifactual increase in activity on the attenuation corrected images secondary to the high density of the arthroplasty hardware.
Figure 21
Sagittal PD-weighted MR image with fat saturation (A) shows an expansile bone marrow replacing lesion in the proximal humeral diaphysis (asterisk) with periosteal reaction and adjacent soft tissue oedema in keeping with osteosarcoma. Avid post contrast enhancement is not shown. Lateral radiograph of the humerus (B) shows postsurgical changes status post humeral resection and megaprosthesis placement with shoulder and elbow arthroplasty. Follow-up F-18 FDG PET image (C) shows increased radiotracer activity surrounding the megaprosthesis by the elbow, concerning for recurrent osteosarcoma. Increased activity is noted on both non-attenuation corrected (D) and attenuation corrected (E) images (D, E – arrowheads), confirming true hypermetabolic activity. Recurrent neoplasm was confirmed on histology
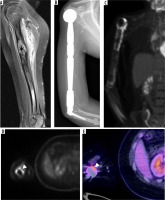
Triceps insufficiency
Instrumentation of the extensor mechanism is required for exposure of the posterior aspect of the joint space for TEA. Various techniques of triceps reflection/splitting have been described [21]. Even in some triceps-preserving techniques, the edges of the triceps tendon are stripped for better visualisation, and chevron osteotomy of the olecranon is performed [1,20,47]. The most common cause of triceps tendon insufficiency is failure of surgical reattachment; however, traumatic tendon rupture is also possible. A single institution review of 867 TEAs identified 16 cases of triceps tendon insufficiency, of which 9 cases where in individuals initially treated for RA and the other 7 for post-traumatic OA [48]. This included patients who underwent triceps-sparing and triceps-splitting approaches. Triceps tendon insufficiency presents on average 3 years after TEA and results in the patient’s inability to actively extend their elbow.
A change in the posterior contour of the elbow with palpable prominence of the implant and atrophy of the posterior soft tissues are encountered on physical examination. Surgical management is based on the health of the olecranon process and triceps tendon. If both are of satisfactory quality, a direct repair is indicated. An anconeus rotational flap can be utilised in the event of a severely atretic triceps. If there is also resorption of the olecranon process, then an Achilles tendon allograft with calcaneal bone block can be considered. Of the 16 cases of triceps insufficiency outlined by Celli, 14 patients reported excellent or good MEPS scores following surgical repair of the triceps mechanism with direct suture (7 elbows), anconeus flap rotation (4 elbows), and Achilles tendon allograft (4 elbows) [48].
MRI and US are excellent in identifying an attenuated or discontinuous triceps tendon (Figure 22). However, US is operator dependent and relies on local expertise.
Figure 22
Sagittal (A) and axial (B) PD-weighted MR images demonstrate discontinuity of the triceps tendon (A and B – arrowheads). Pre-operative (C) and post-operative (D) lateral elbow radiographs of the same elbow status post triceps tendon Achilles allograft repair; in (D) note the increased thickness and density of the soft tissues posterior to the elbow (C, D – white dashed lines) at the level of the hardware flange
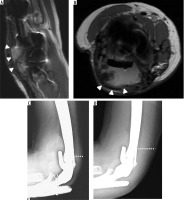
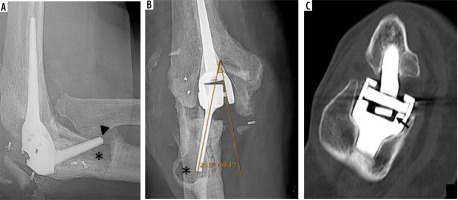
Polyethylene bushing wear
If the components are not satisfactorily aligned, increased wear of the polyethylene bushing can occur and result in subjective and objective instability [49]. Rates of bushing wear are low, with 1.3% of 919 TEA reported by Lee et al. [50]. Modern TEAs have the ability to exchange the articular bushings, which carries lower morbidity than total revision arthroplasty.
Bushing wear allows greater varus/valgus angulation of the ulnar component and is diagnosed on anterior-posterior radiographs as an angle greater than 7 degrees between the long axis of the articulating ulnar component and medial or lateral aspect of the humeral articular yoke (Figure 23) [16,49,50].
Figure 23
Lateral (A) and frontal (B) radiographs of the elbow demonstrate marked osteolysis about the ulnar stem (A, B – asterisk) in keeping with histology-proven particle disease. Note cortical deficiency of the anterior ulnar cortex (A – arrowhead) and extraosseous extension of the hardware. Eccentric positioning of the articulating component of the ulnar hardware with respect to the humeral articular yoke is seen on frontal radiographs (B) with elevated angle between the 2 components (21 degrees, normal < 7). This is consistent with polyethylene bushing wear. Coronal reformatted CT image (C) demonstrates polyethylene bushing wear in another patient (arrow). Note that some varus-valgus angulation is expected in the “sloppy hinge” of the current TEA designs
Conclusions
Elbow arthroplasty is a viable option for improving pain control and range of motion in patients with severe disability and in those refractory to more conservative treatment. Complication rates have decreased secondary to improved component design and surgical technique, fuelled by a more complete understanding of elbow anatomy and kinematics over the last 50 years. Knowledge of elbow arthroplasty hardware, technique, and indication helps the radiologist make an accurate diagnosis and convey valuable information for surgical management in cases of suspected complication.